Critical Care POCUS: Assessing Your Sickest Patients
Nick Kelly MD*, Matthew VandeHei MD*, Tessa Damm MD^, Michael Macias MD+, Matthew Riscinti, MD*
*Denver Health Emergency Ultrasound, ^University of Wisconsin School of Medicine & Public Health, +SoCal Medical Education Consortium
Reviewer: Garrett Ghent, MD - Advanced Resuscitation and Ultrasound Stony Brook University Hospital
Ultrasound is a critical component during the evaluation of patients with undifferentiated shock or critical illness.
POCUS can allow you to understand the physiology of the critically ill, instantly identify life-threatening diagnoses, and guide your resuscitations and procedures to help your sickest patients. Multiple studies have demonstrated that ultrasound can aid in narrowing the differential and identifying of the type of shock compared to traditional evaluation (1,2,3,4). Several protocols have been developed to help guide the use of ultrasound in patients presenting critically ill or with signs of shock. A few well known protocols developed by various specialities include: ACES: Abdominal and Cardiothoracic Evaluation with Sonography in Shock (5), GUCCI: Global Ultrasound Check for the Critically Ill (6), and the RUSH Exam: Rapid Ultrasound for Shock and Hypotension (7), which is the protocol we will discuss further. The RUSH protocol uses the mnemonic HI-MAP to guide the user through the required views.
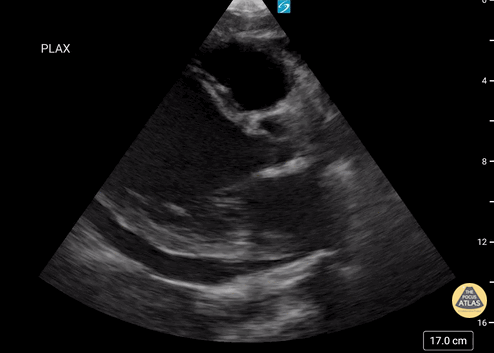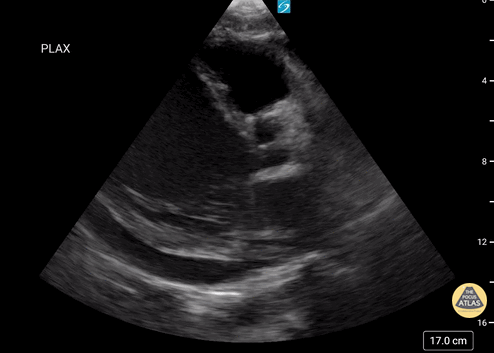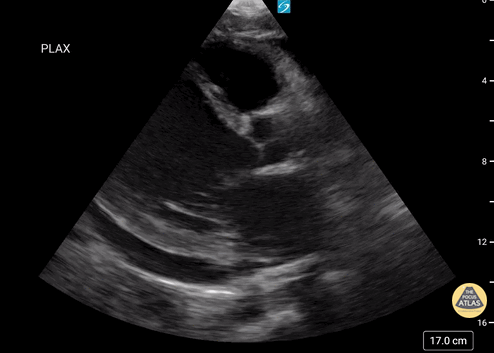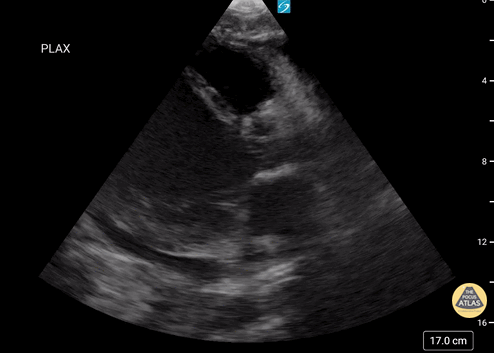
Scanning Locations and Probe Orientation for the RUSH Exam
Algorithmic Approach to Utilizing the RUSH Exam
HI-MAP: Heart
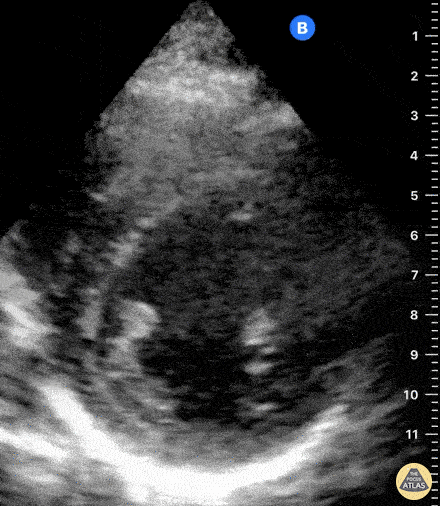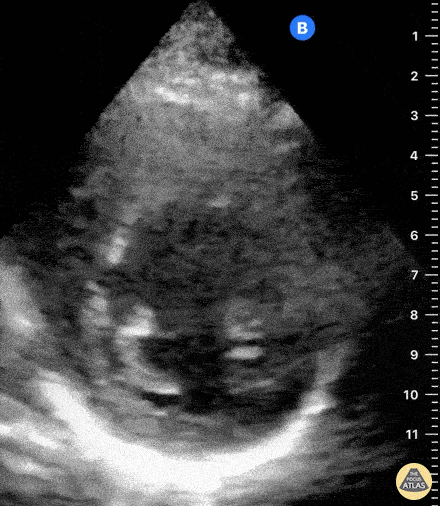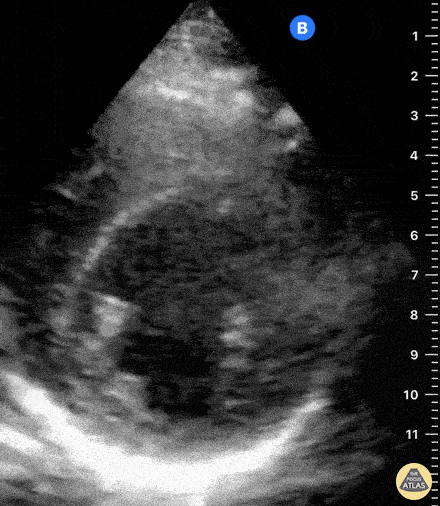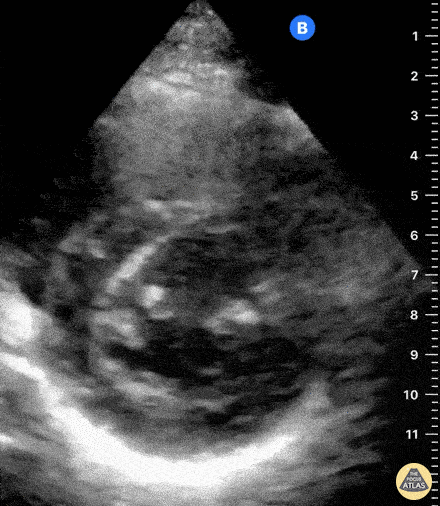
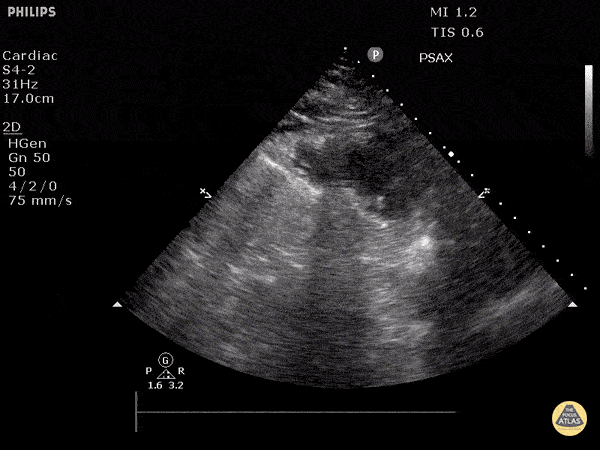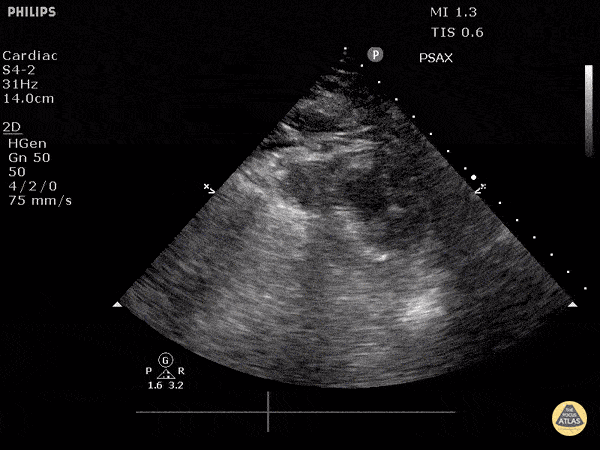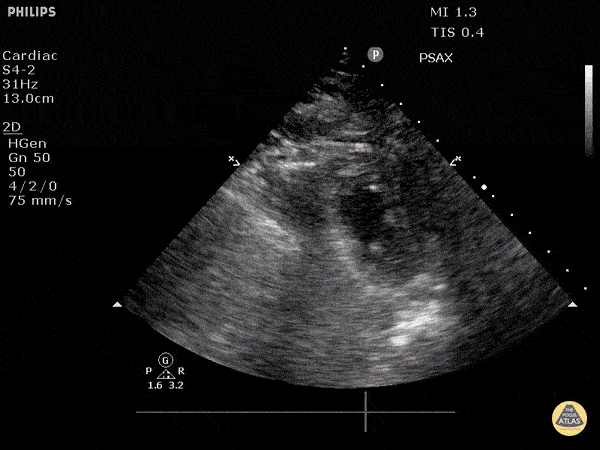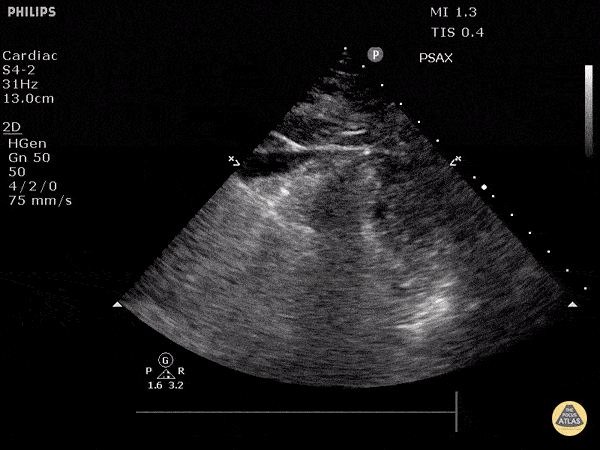
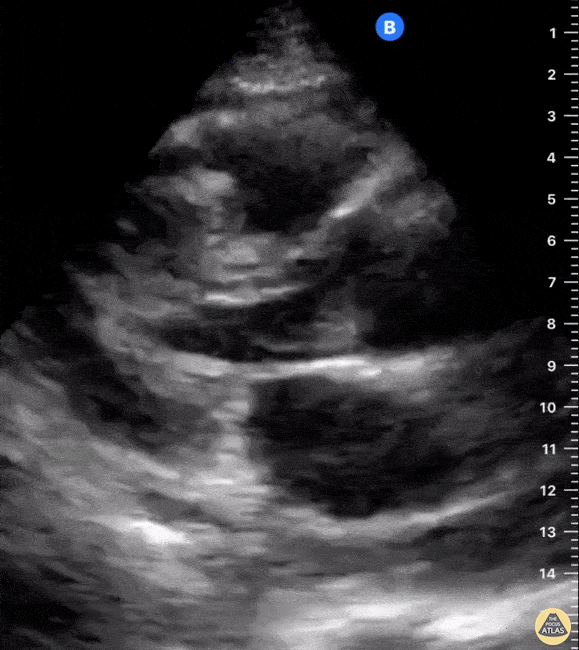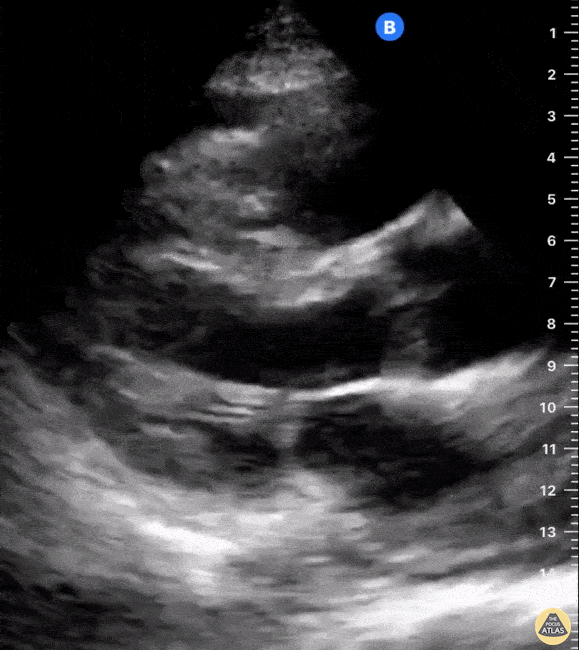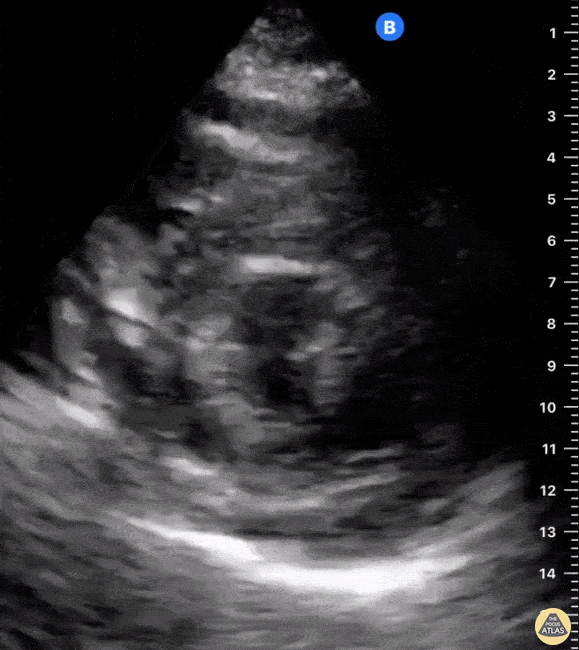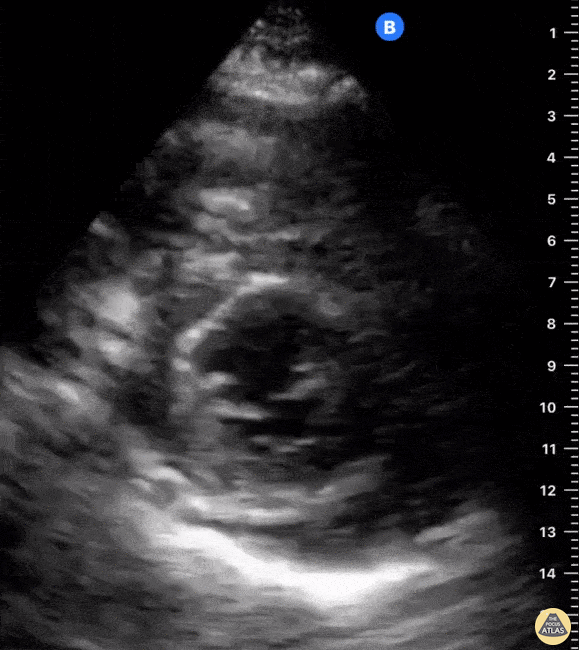
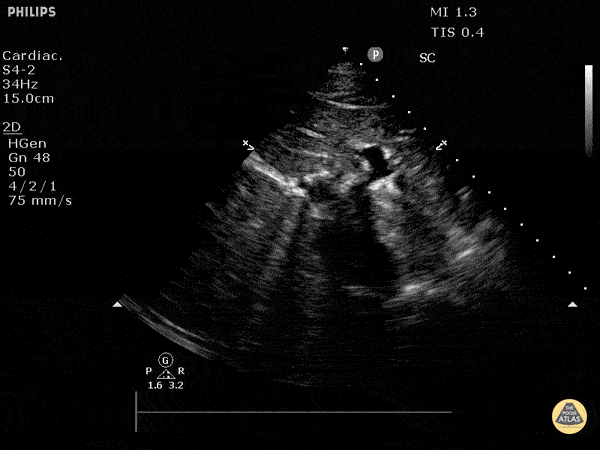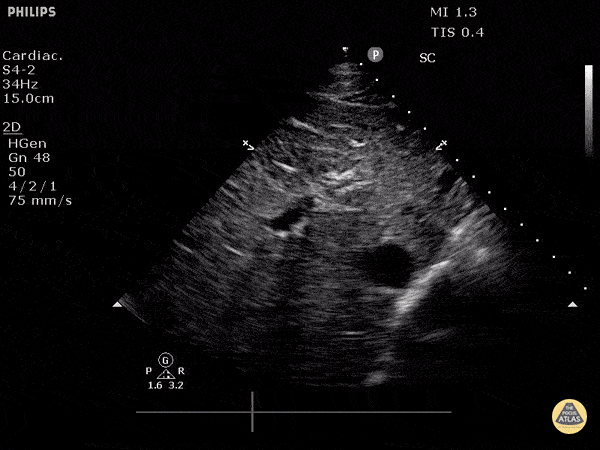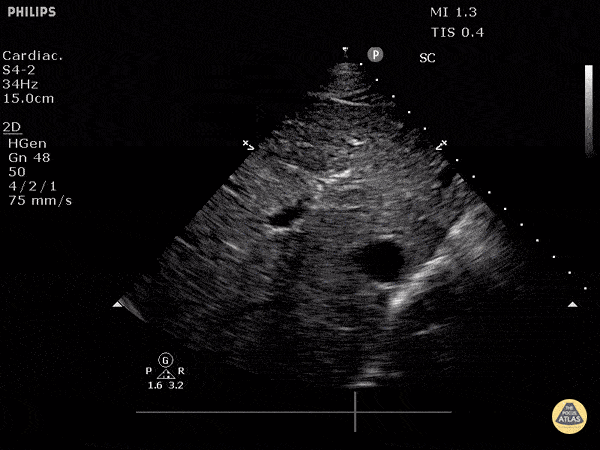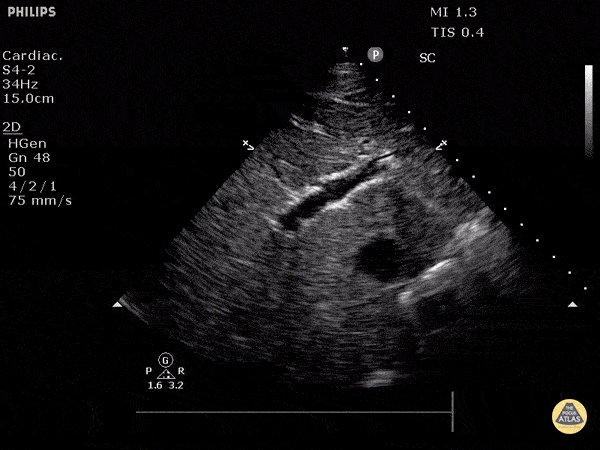
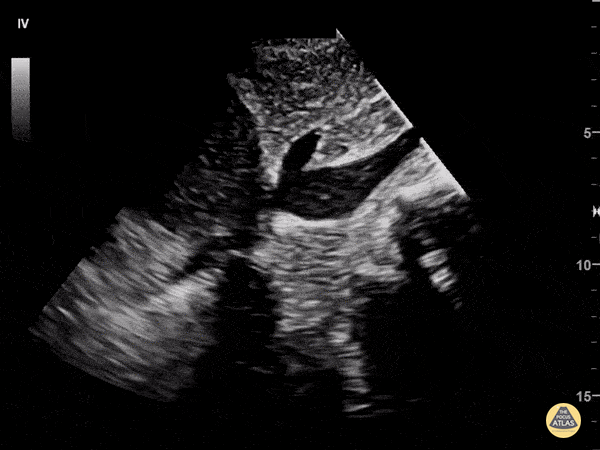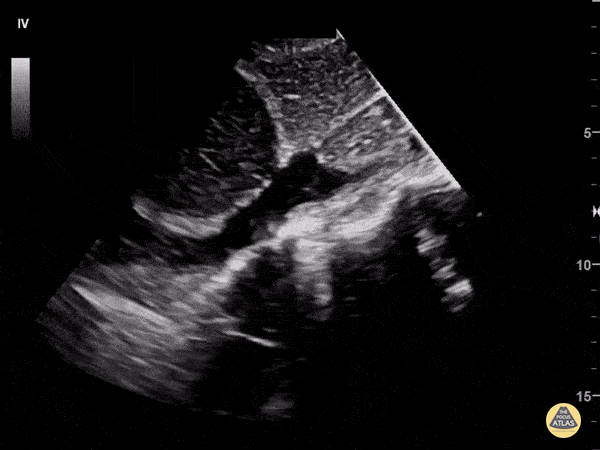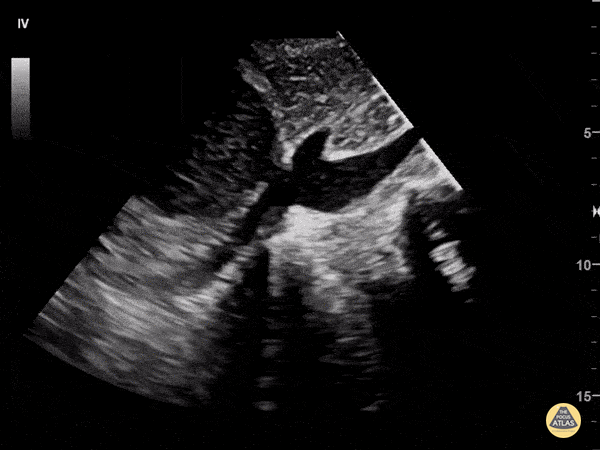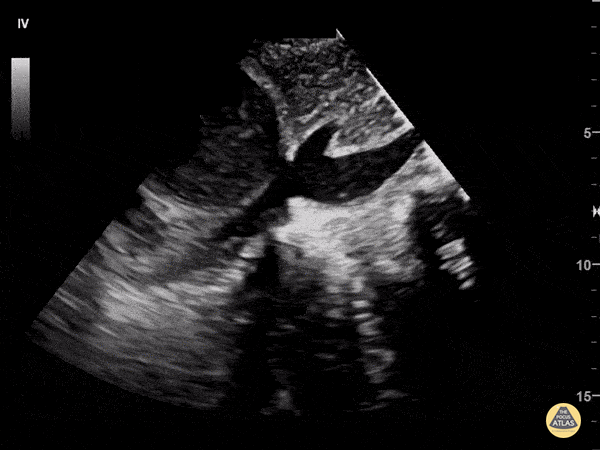
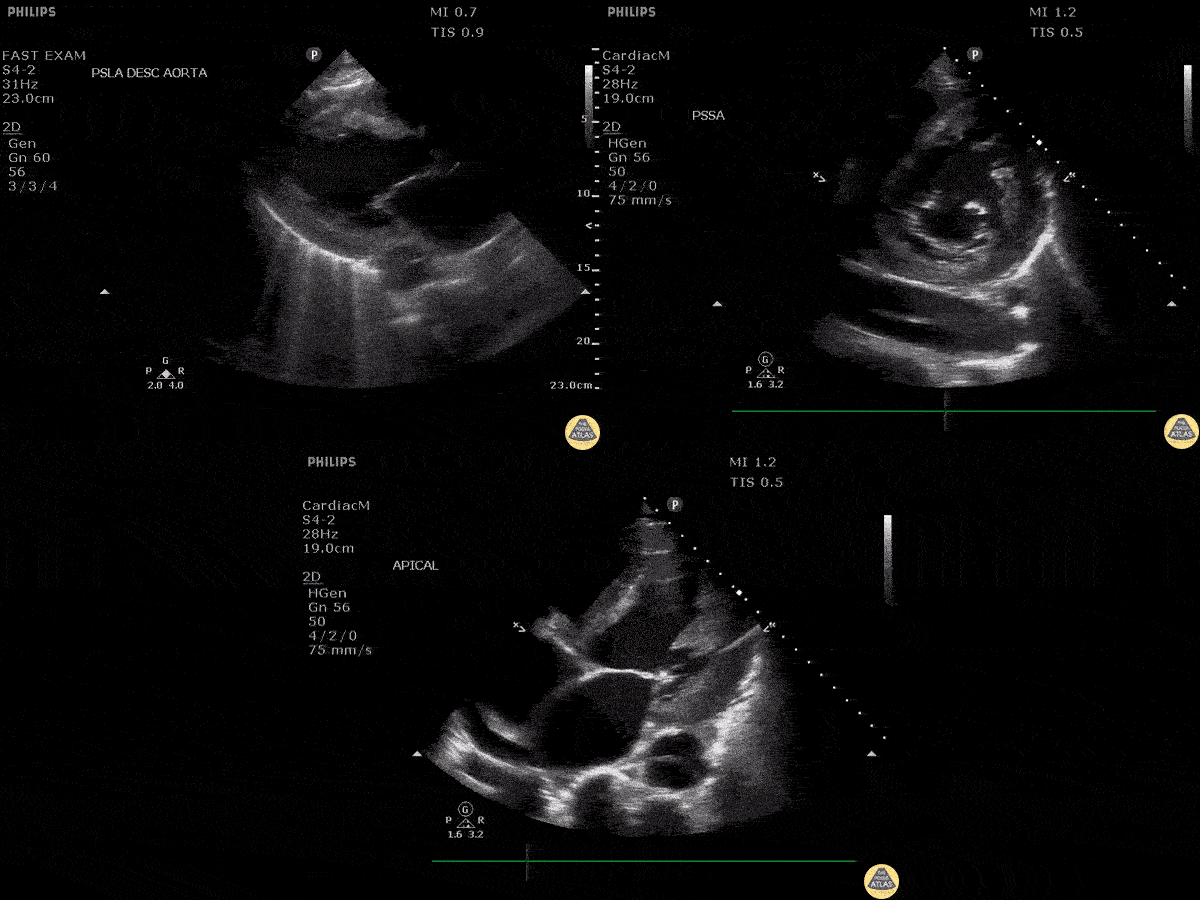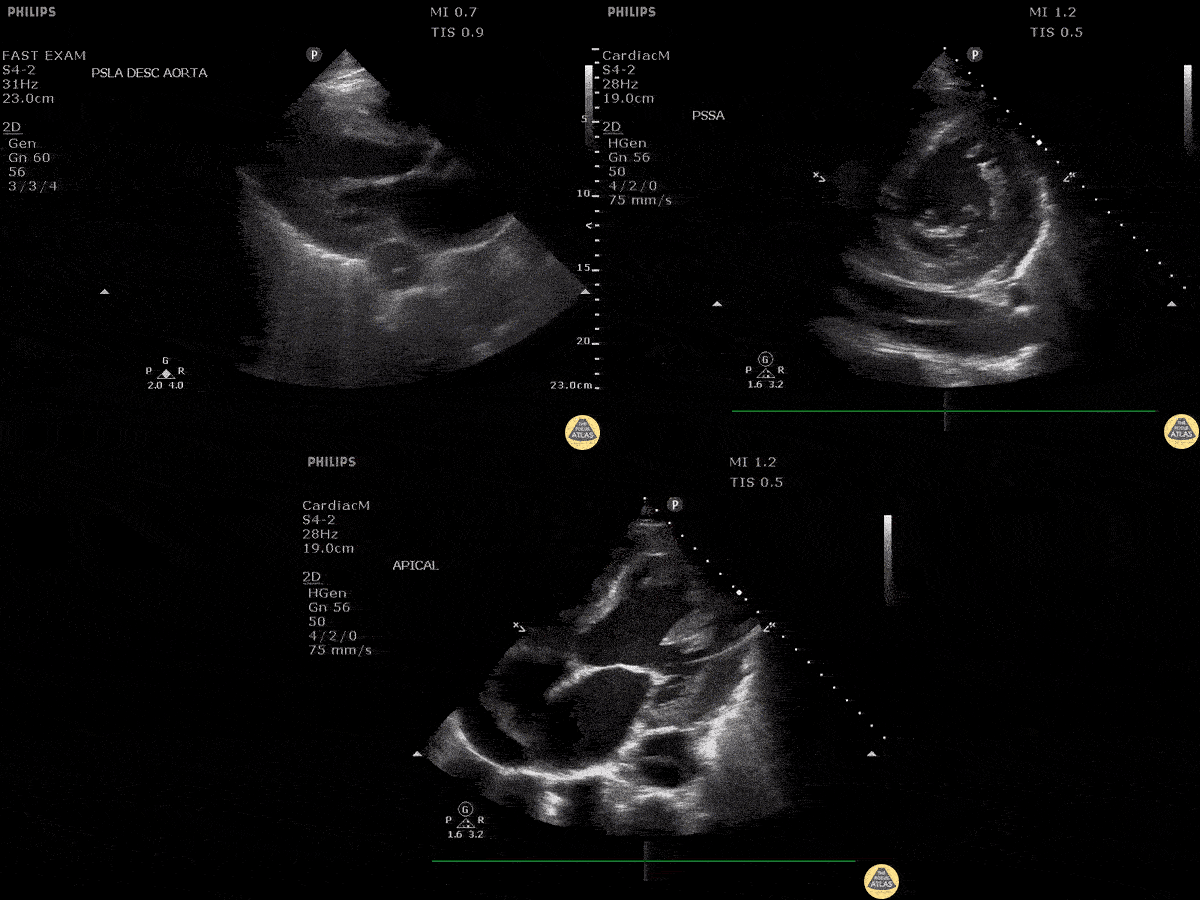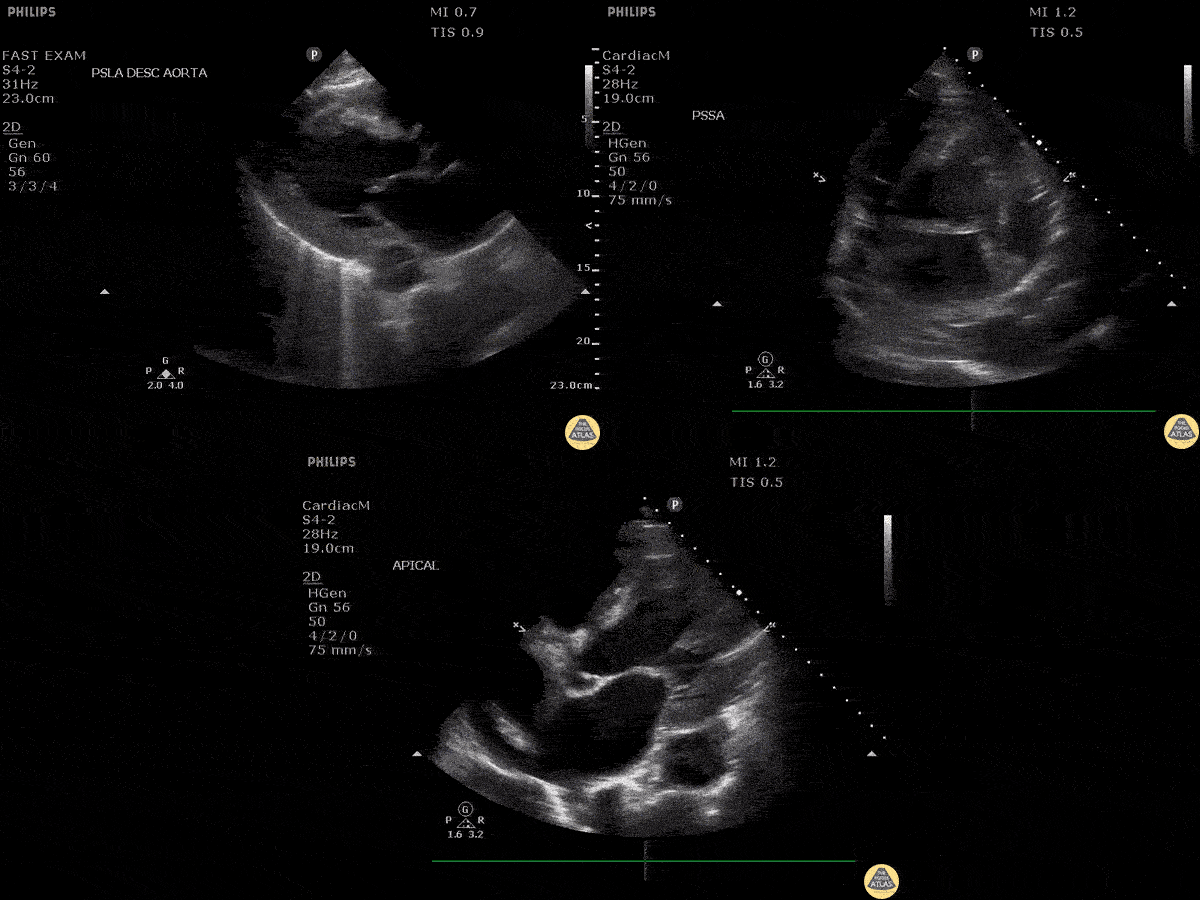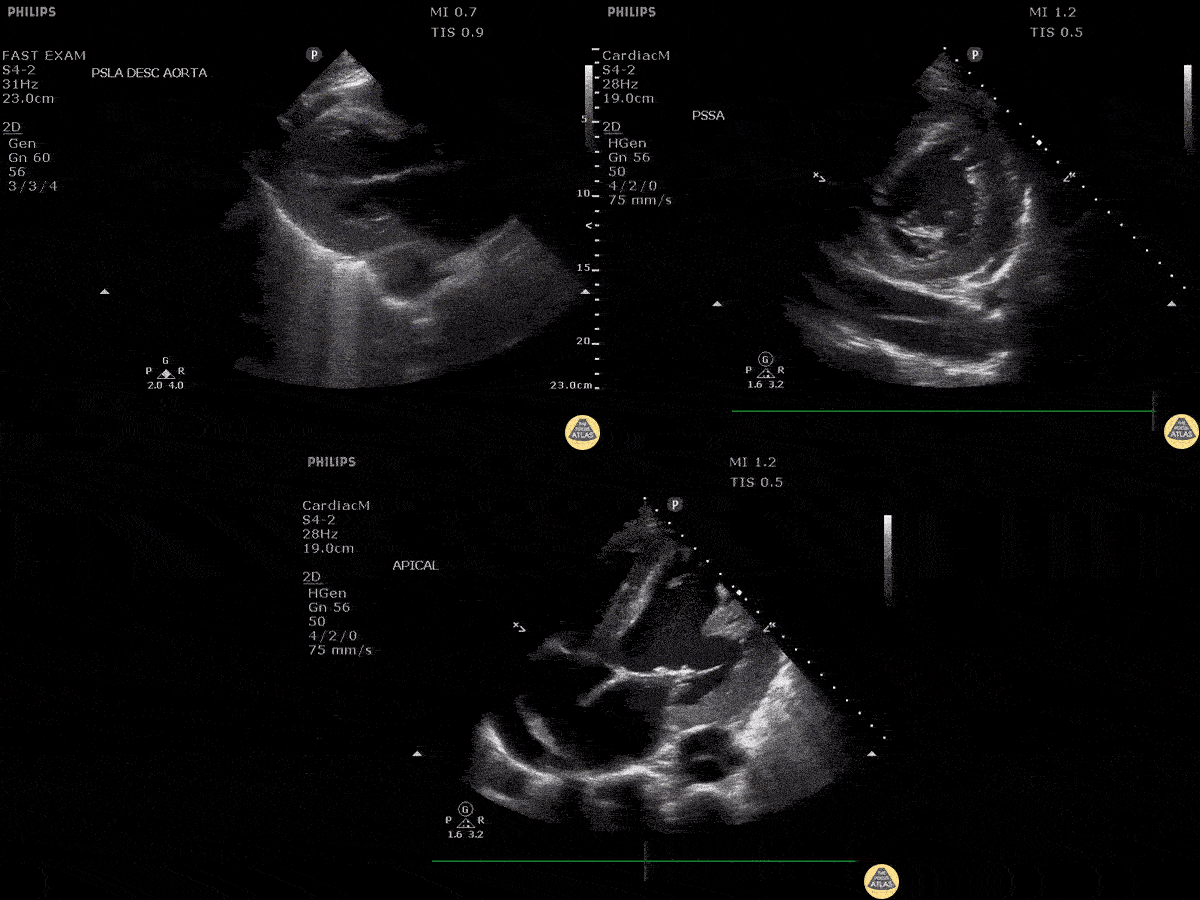
Assessment of the heart includes four views: parasternal long, parasternal short, apical four chamber, and subxiphoid/subcostal. The three main elements to assess for include:
Left ventricular function/contractility
Right ventricular dysfunction
Pericardial effusion with or without evidence of tamponade
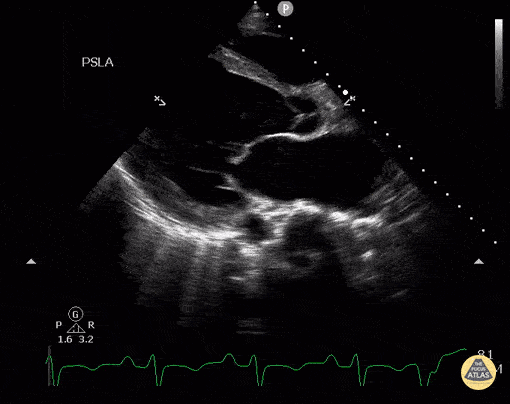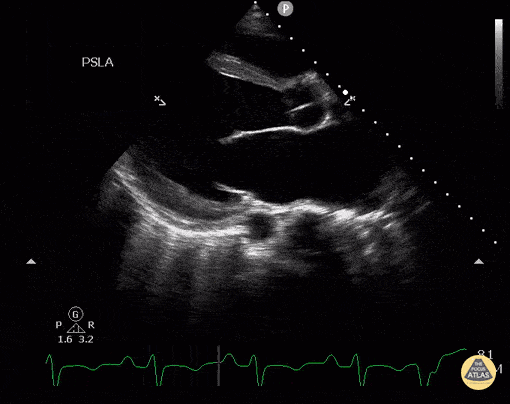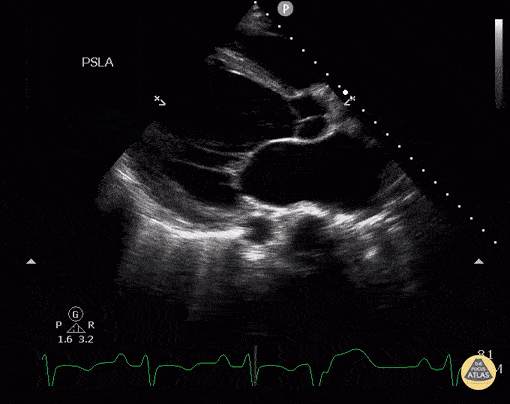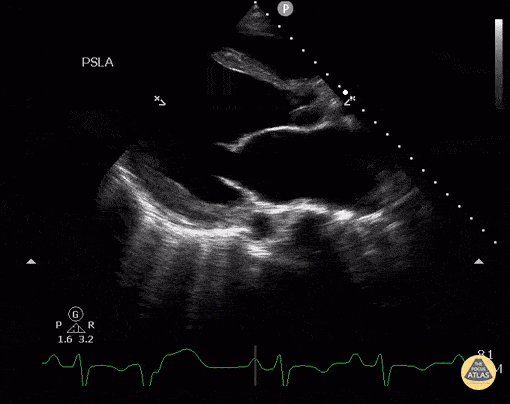
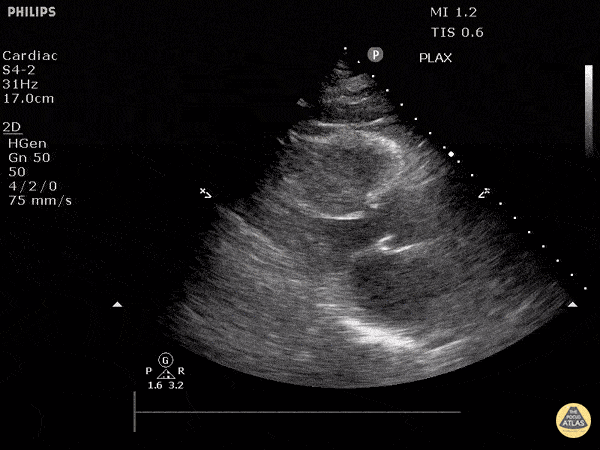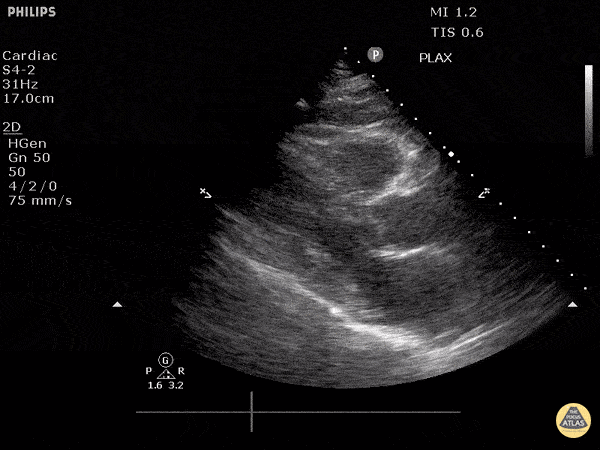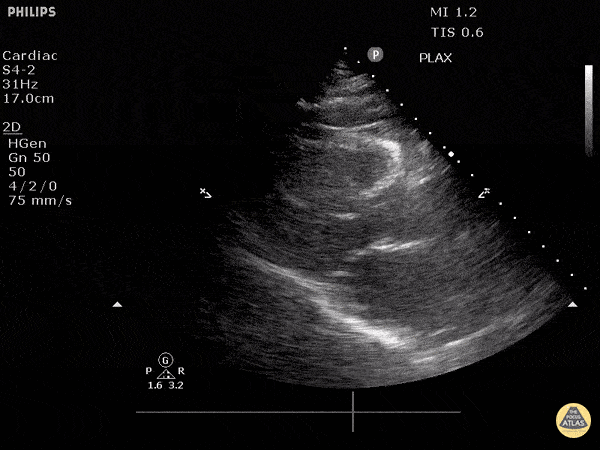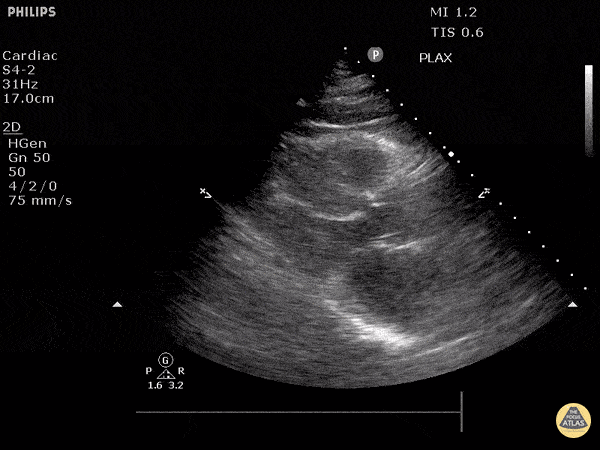
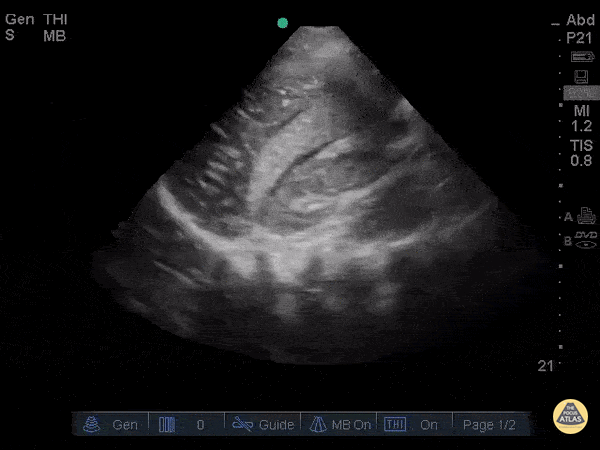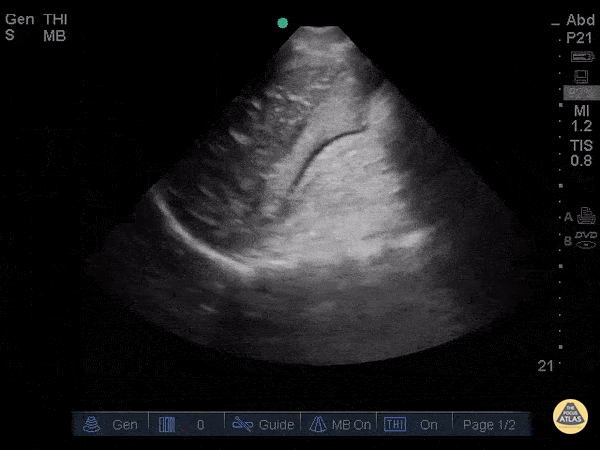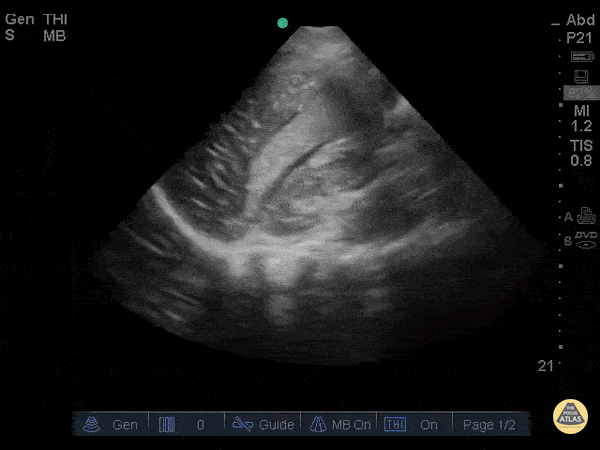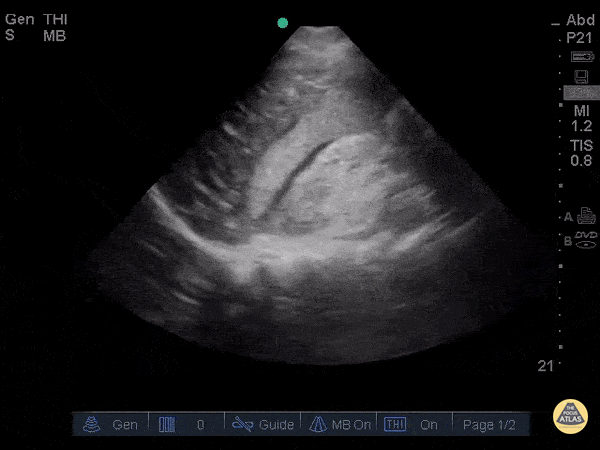
Assessment of Left Ventricular Function
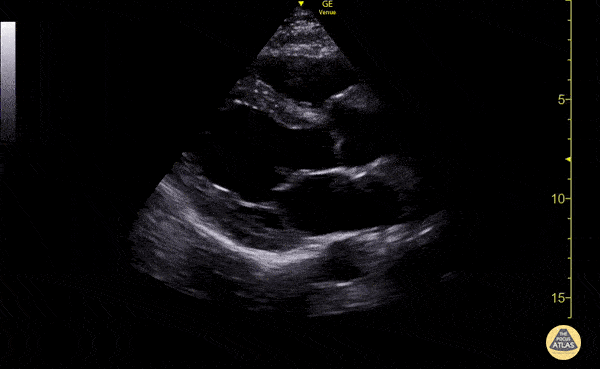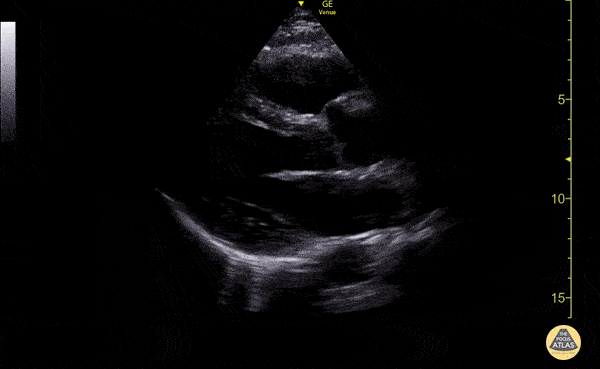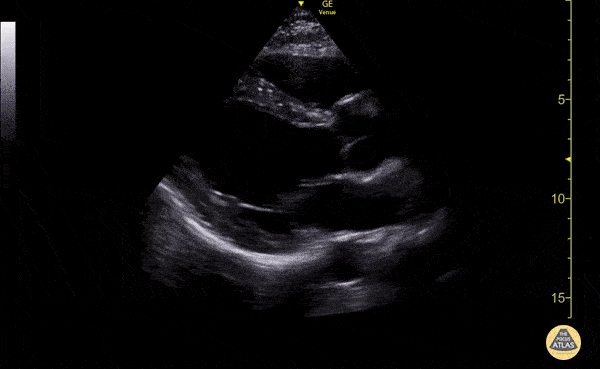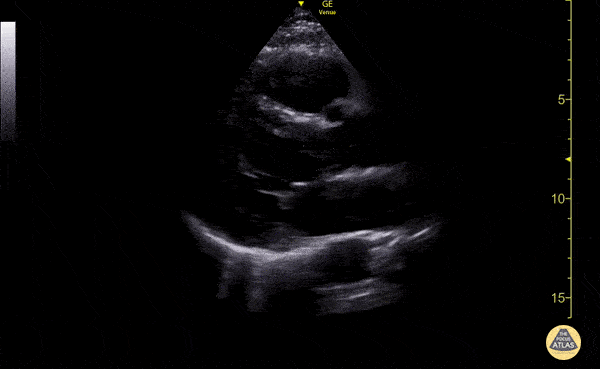
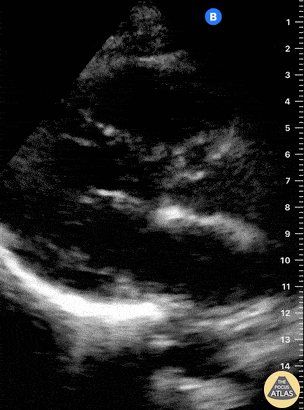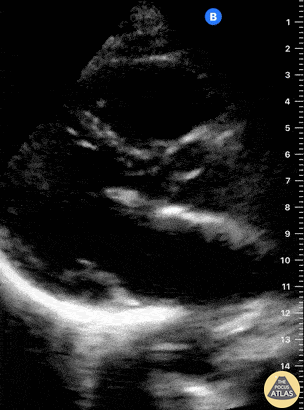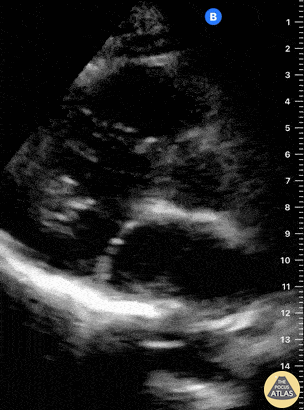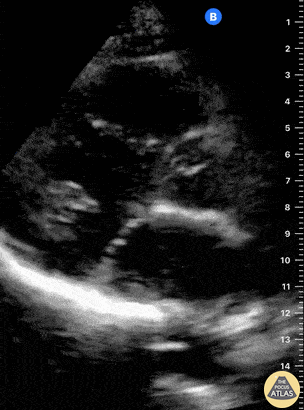
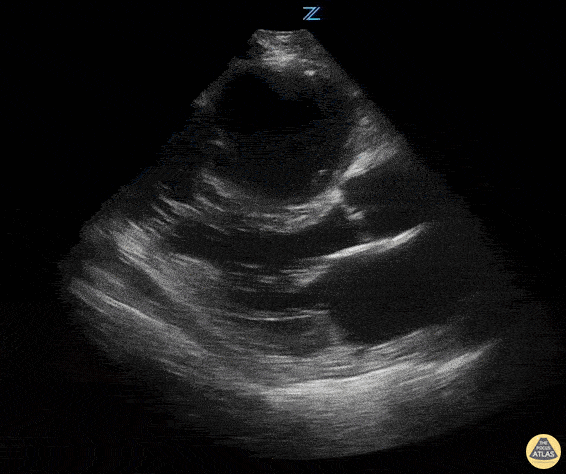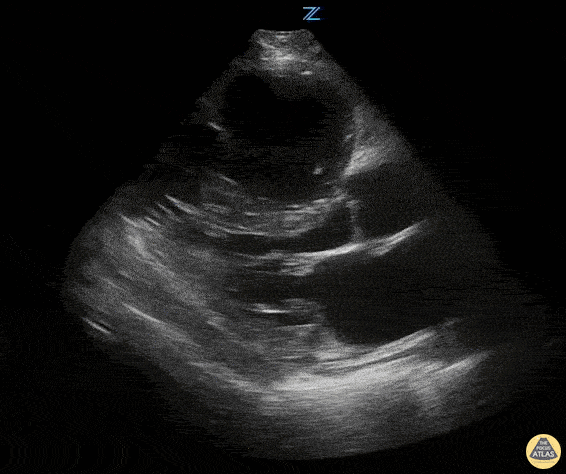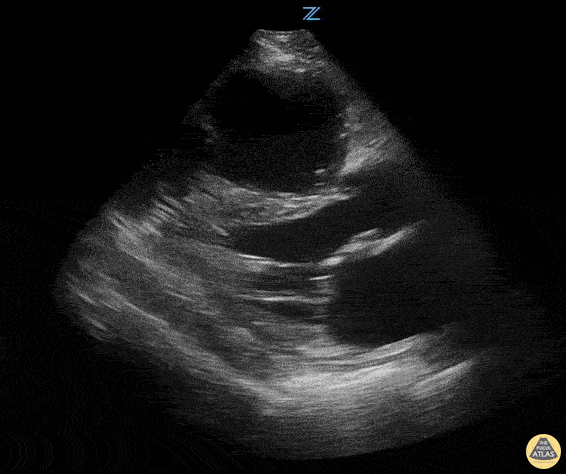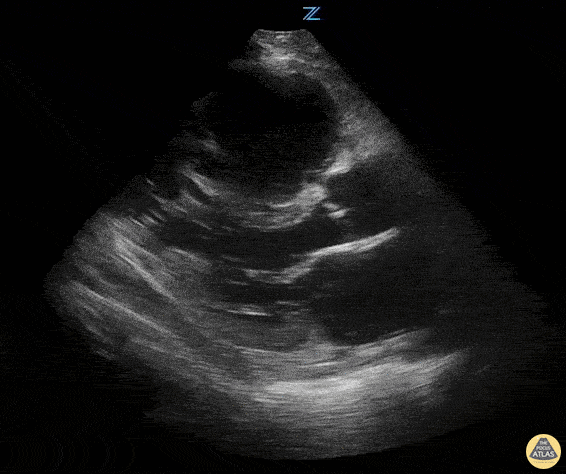
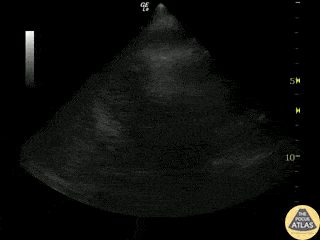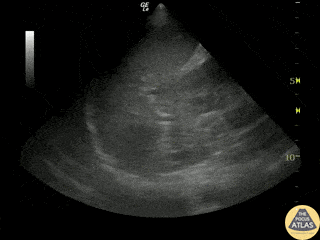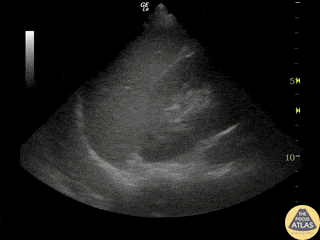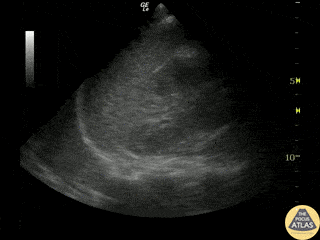
Left ventricular (LV) function can be evaluated in all 4 views but is generally easiest in the parasternal long axis view. It can be measured either by visual estimation or by measurement of the EPSS (end point septal separation). Multiple studies have shown adequate concordance between EM providers and cardiologists in global cardiac function estimation (8,9).
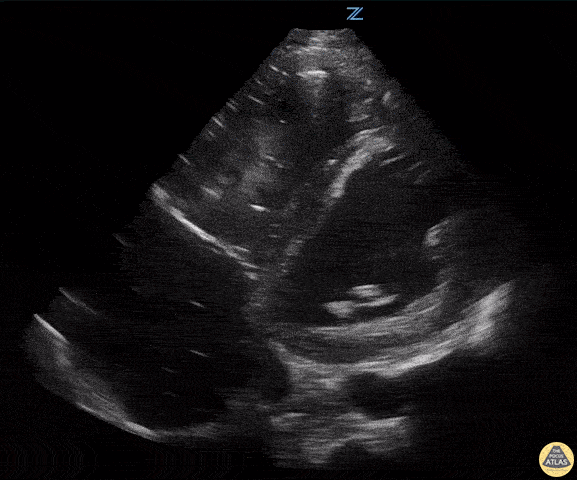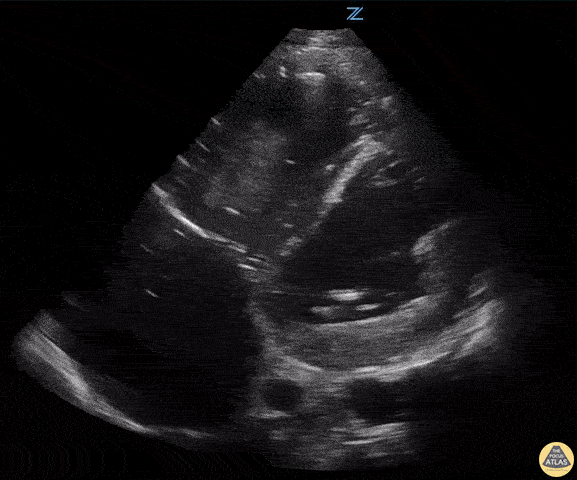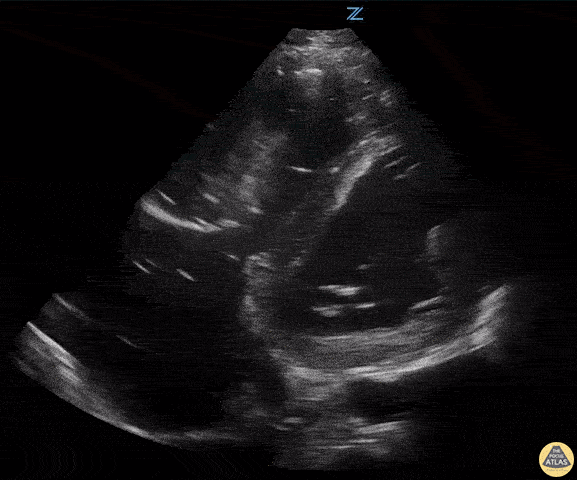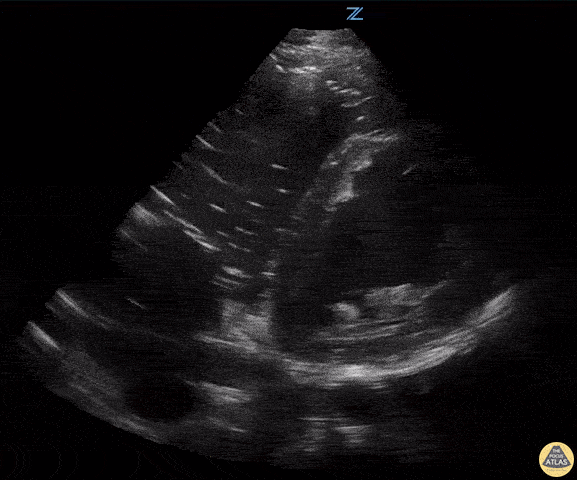
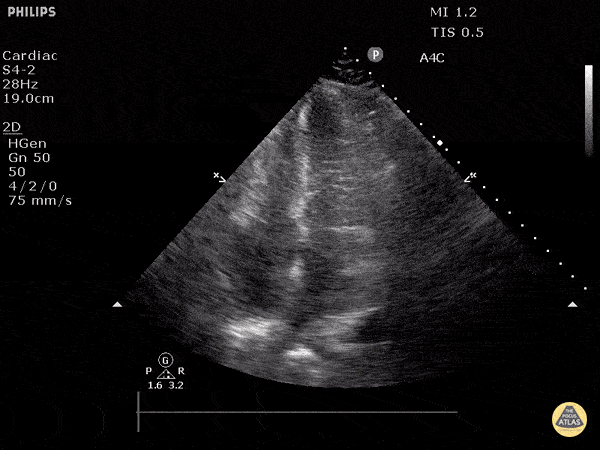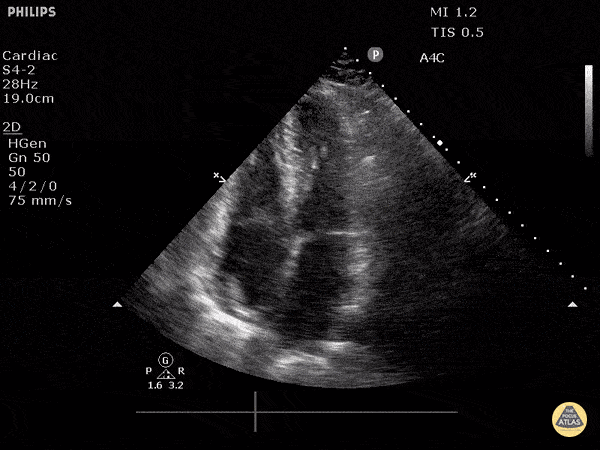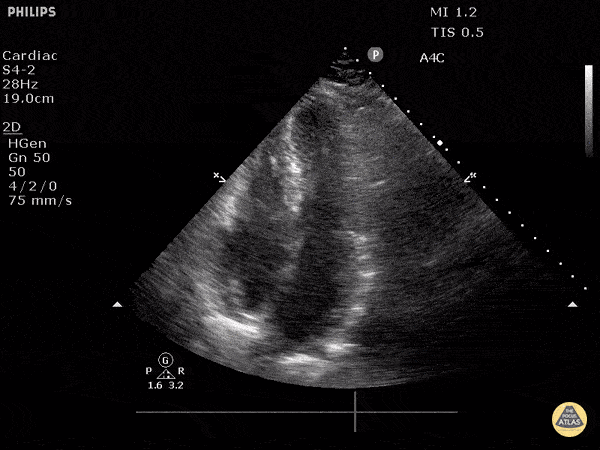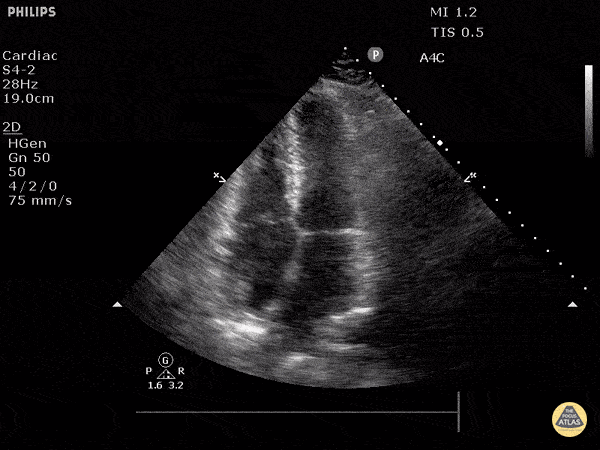
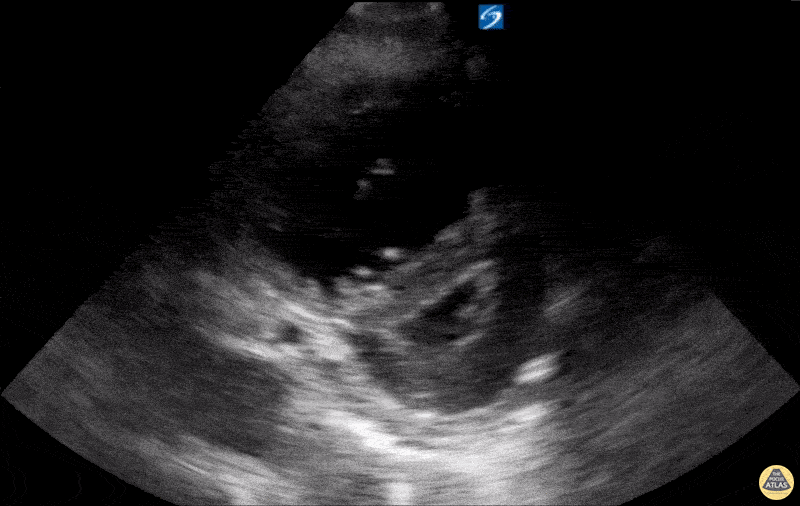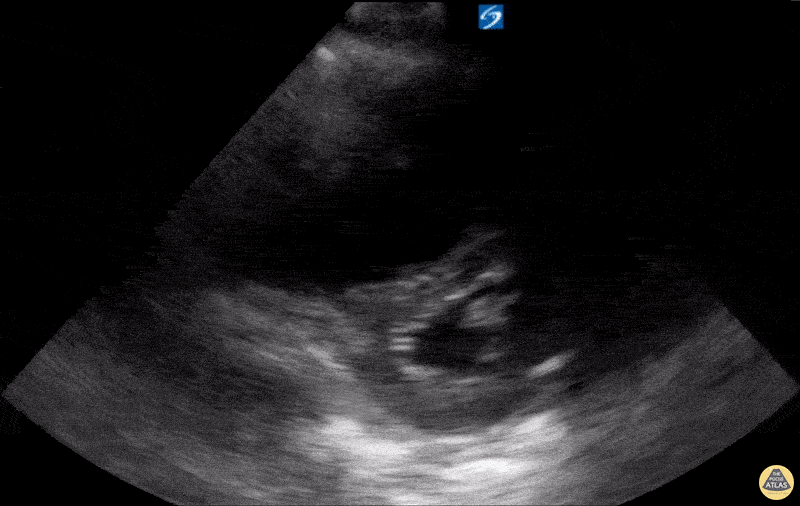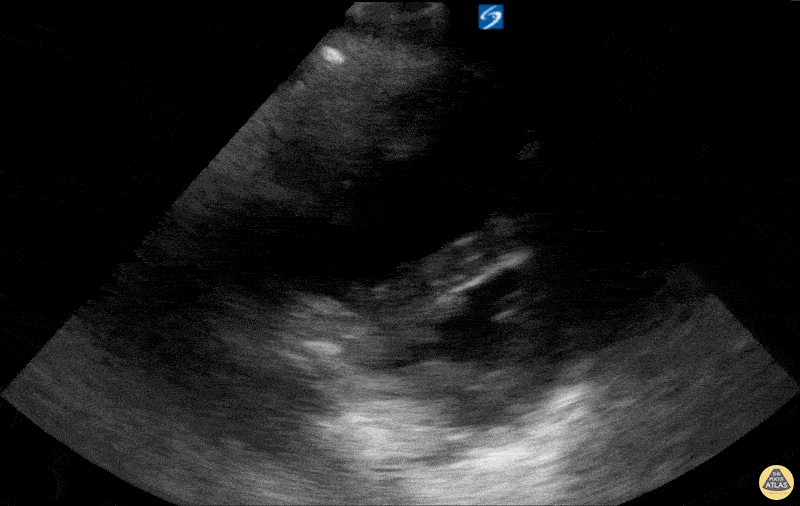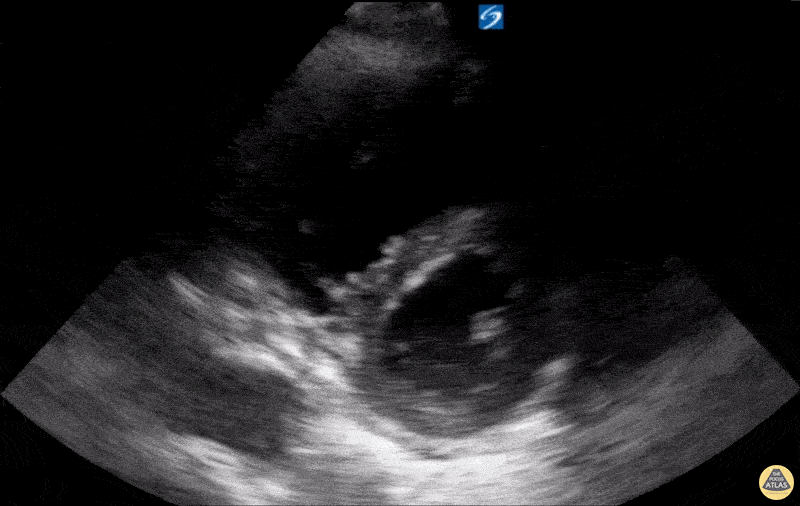
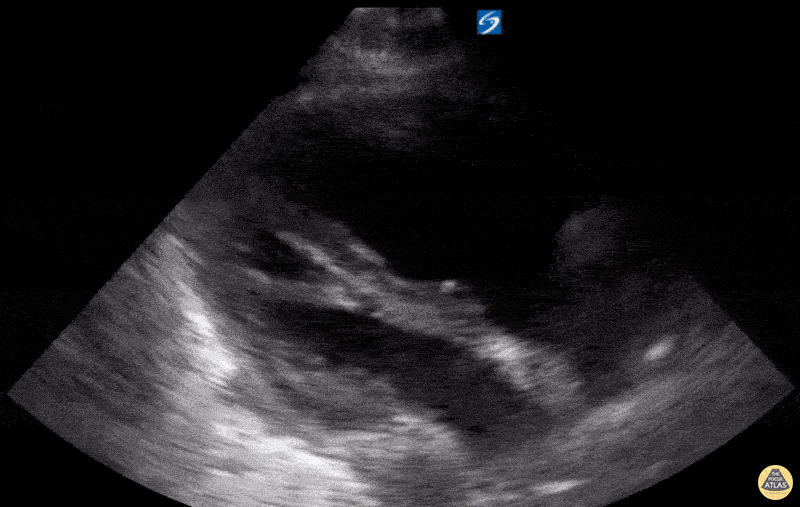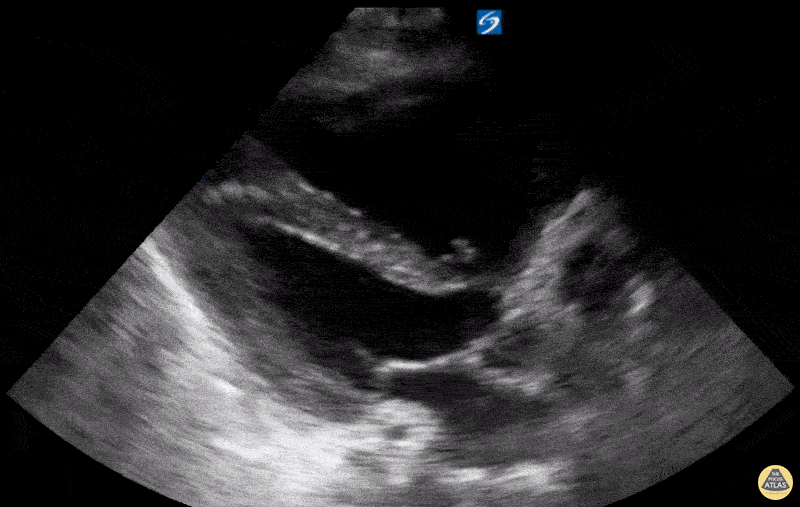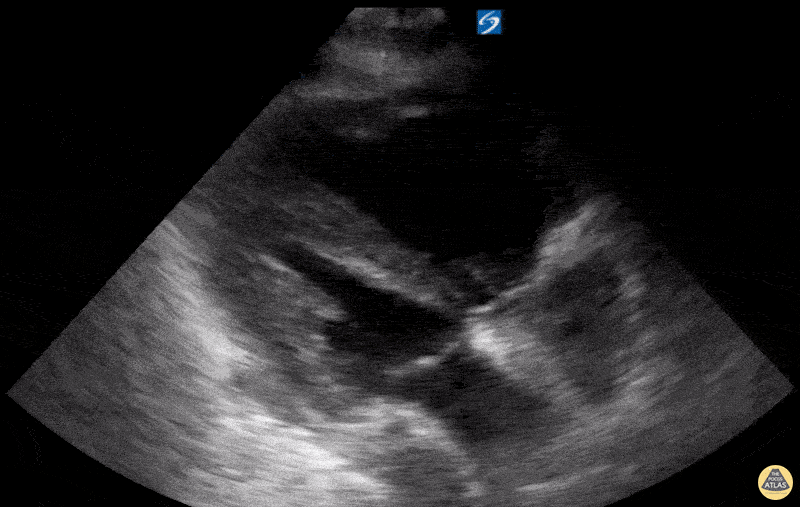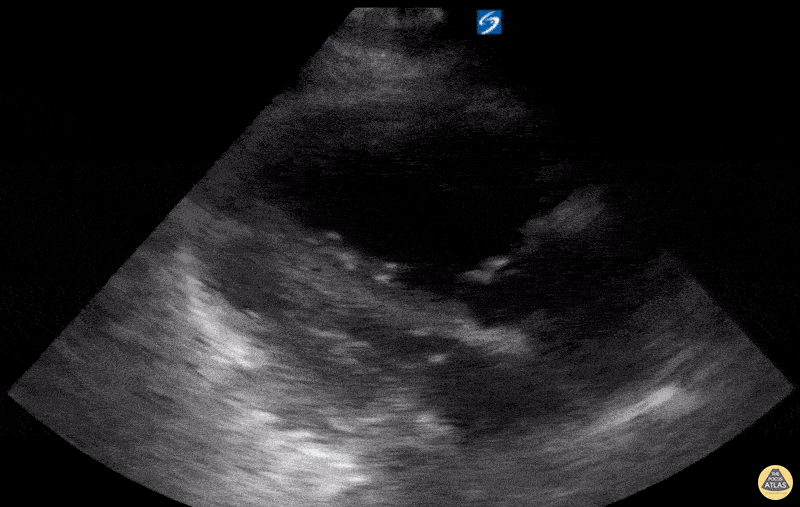
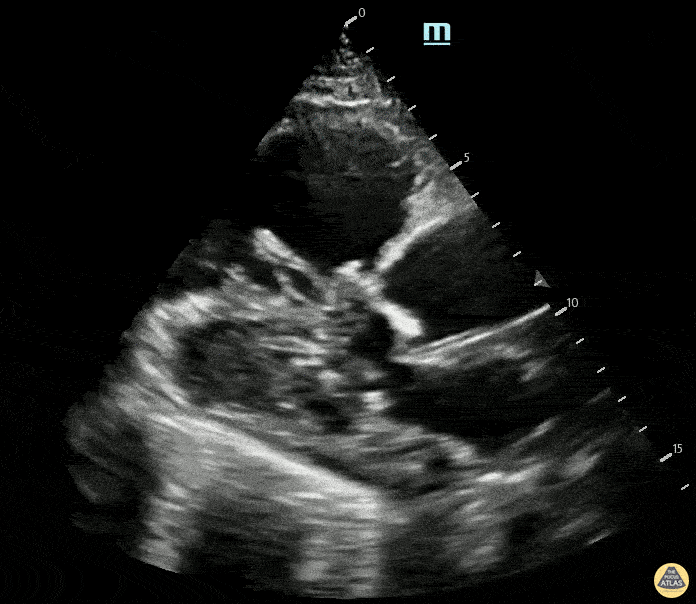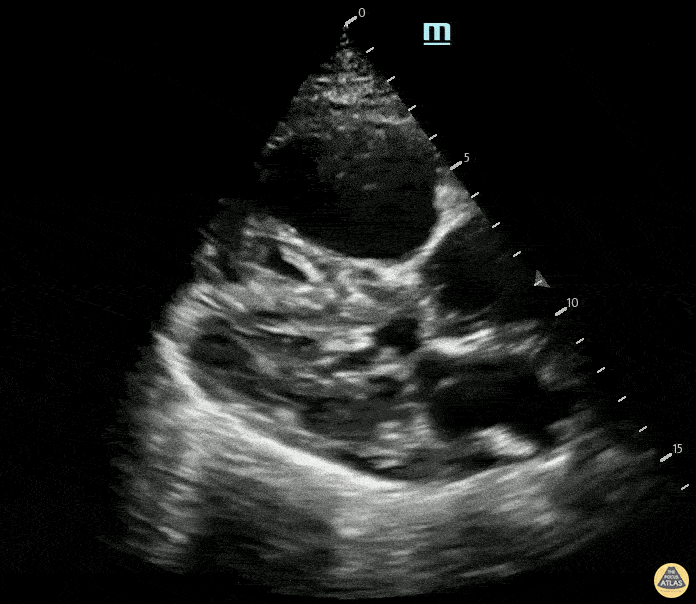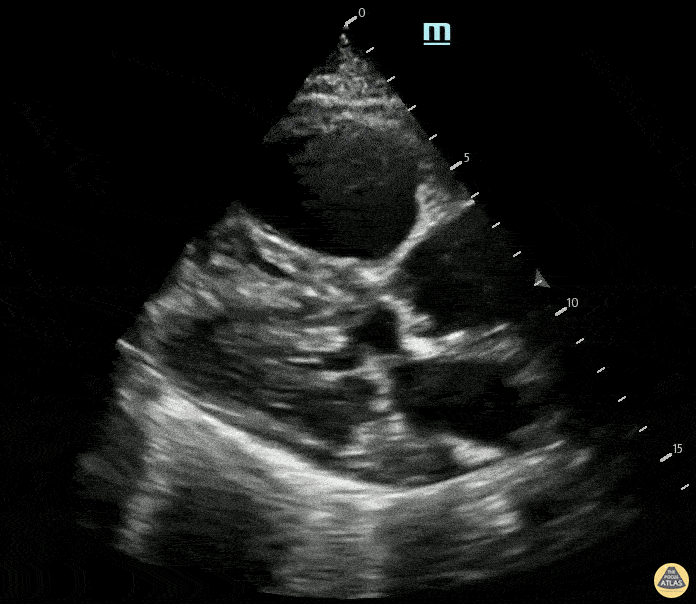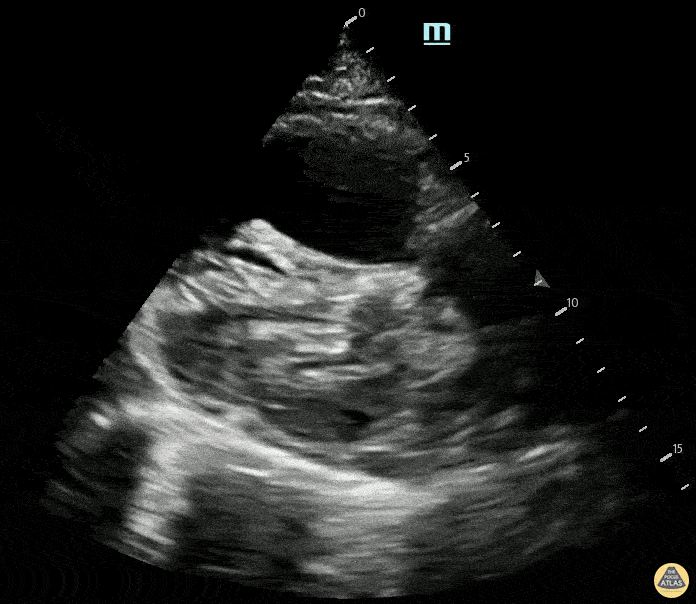
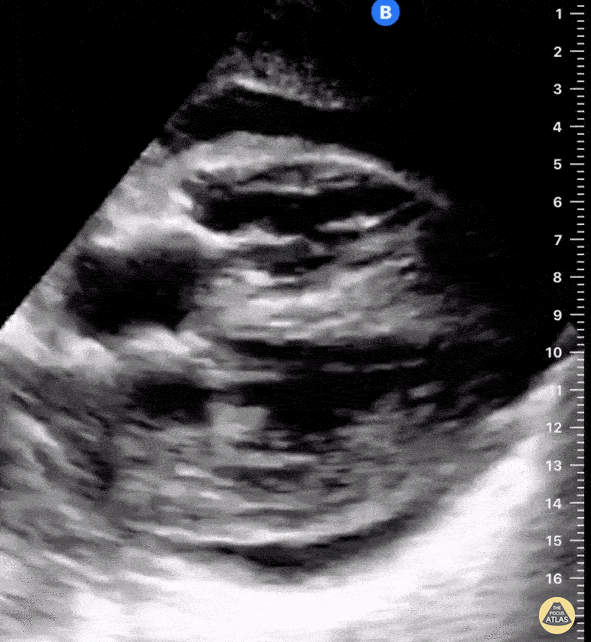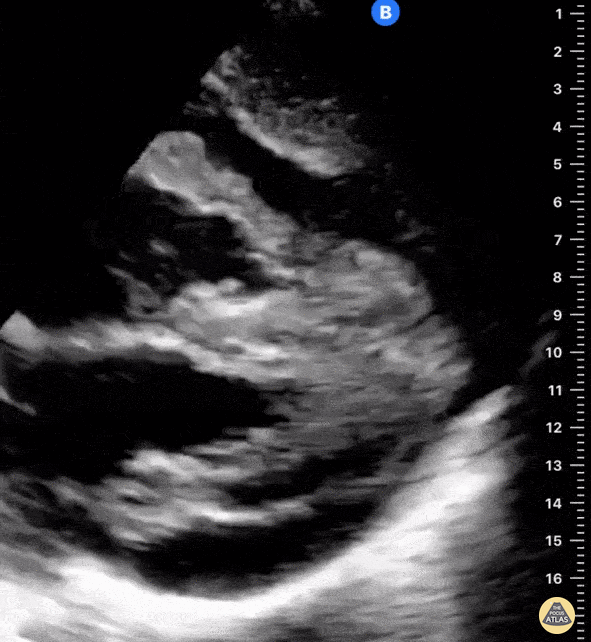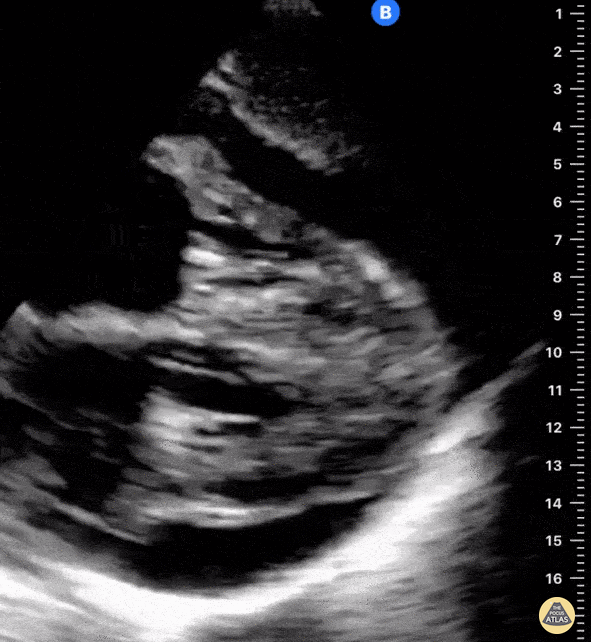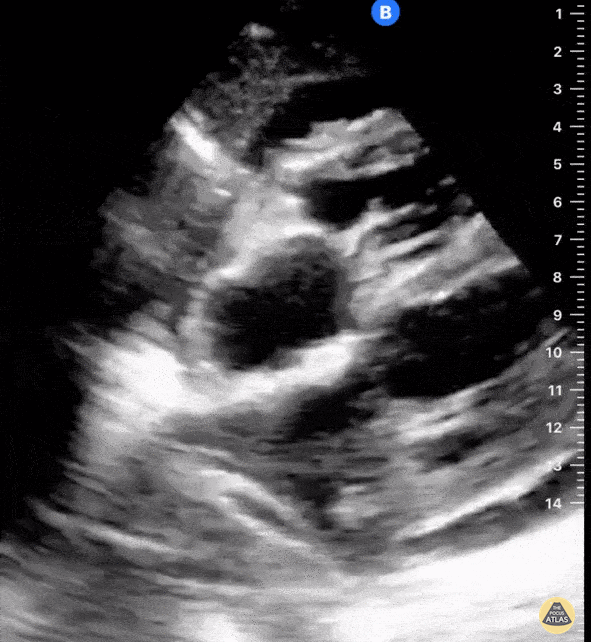
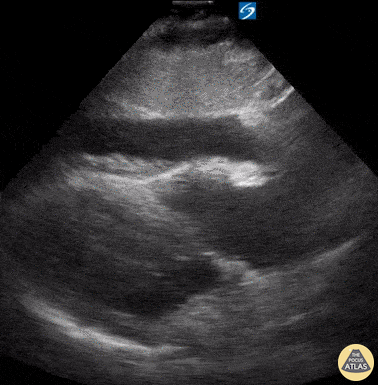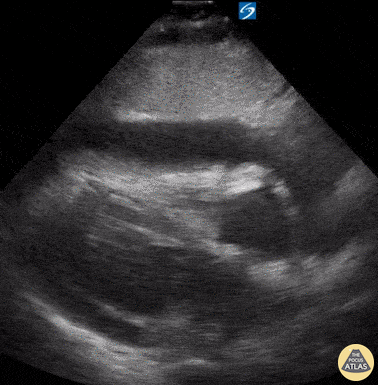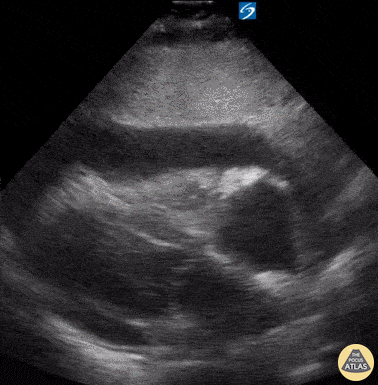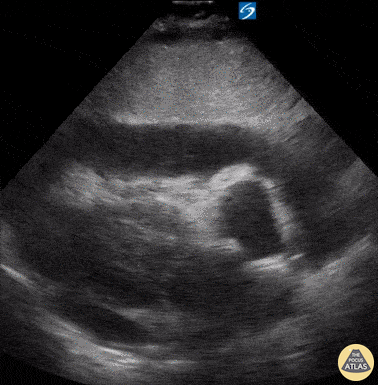
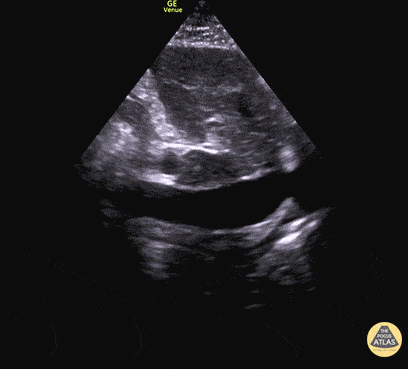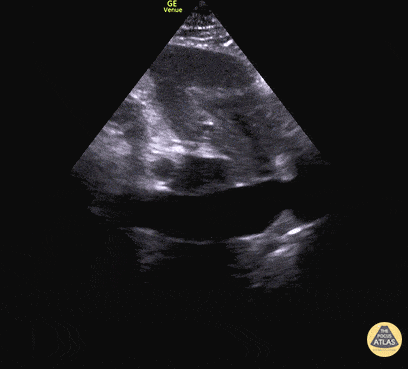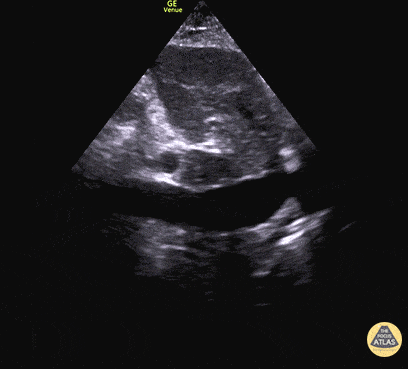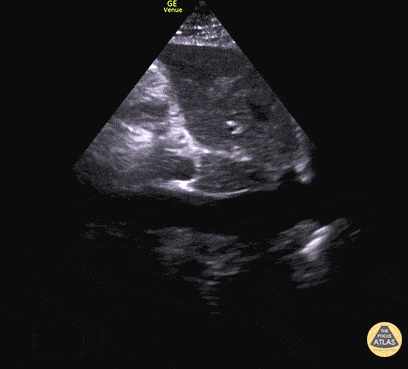
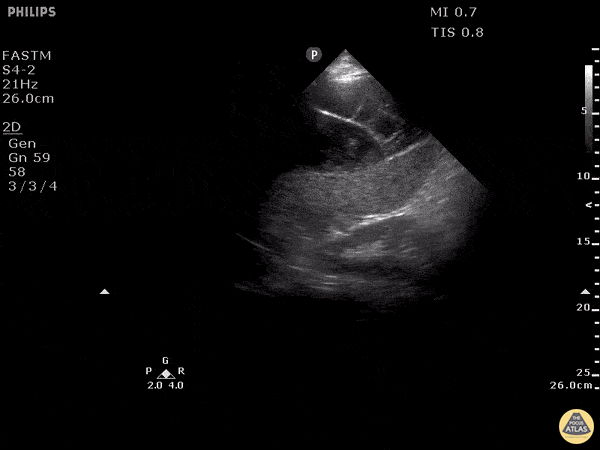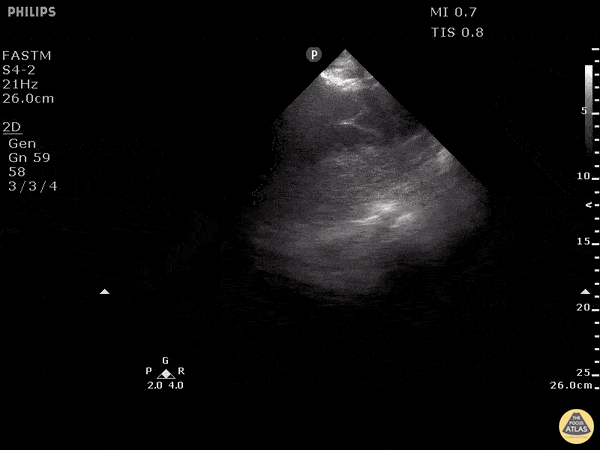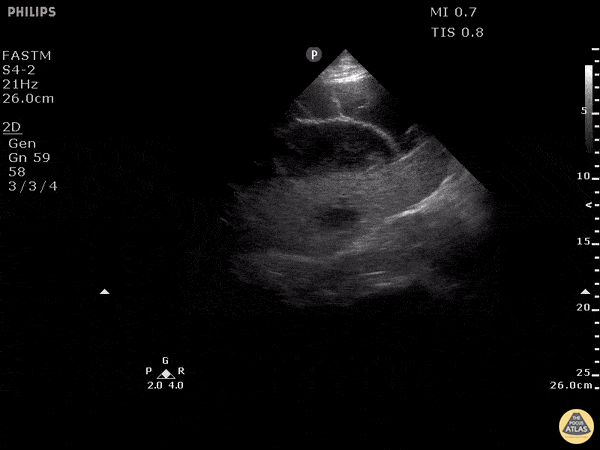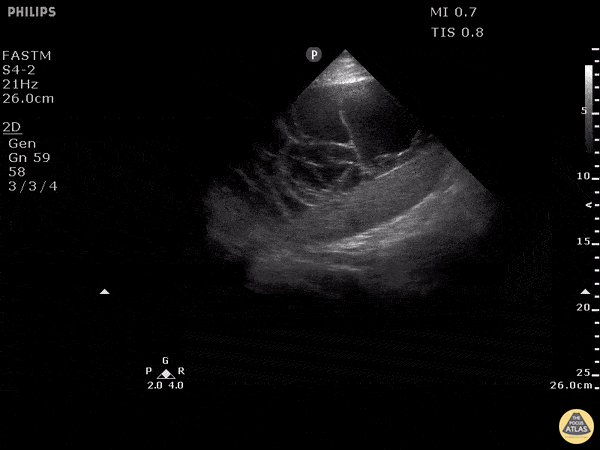
For the RUSH exam, we generally look to break down LV function into broad categories including hyperdynamic, normal, or diminished, with lesser focus on the exact ejection fraction. In general some quick findings that indicate decreased LV function include: left ventricular chamber size not decreasing by roughly a third; the myocardium not thickening during systole; and the anterior leaflet of the mitral valve not coming near the septum during diastole. This is in contrast to a hyperdynamic heart, where you can see the walls of the ventricle almost totally collapsing and touching during the peak of systole.
Examples of Reduced Ejection Fraction:
Examples of Hyperdynamic Function:
Assessment of Right Ventricular Function
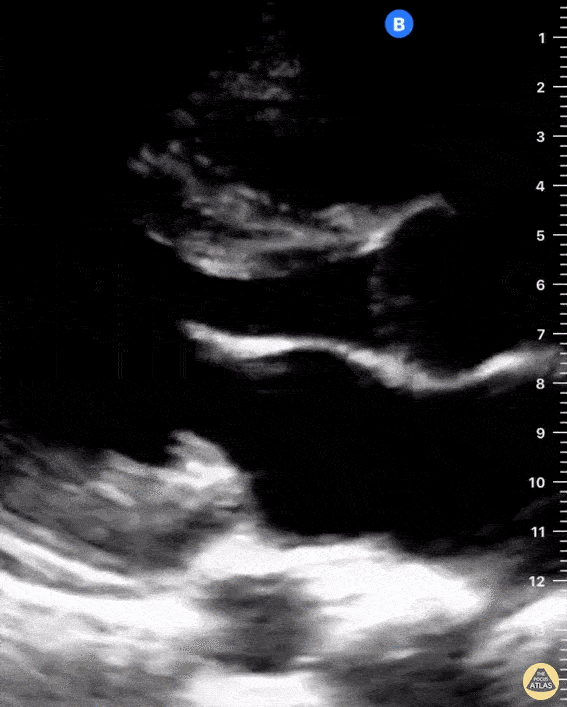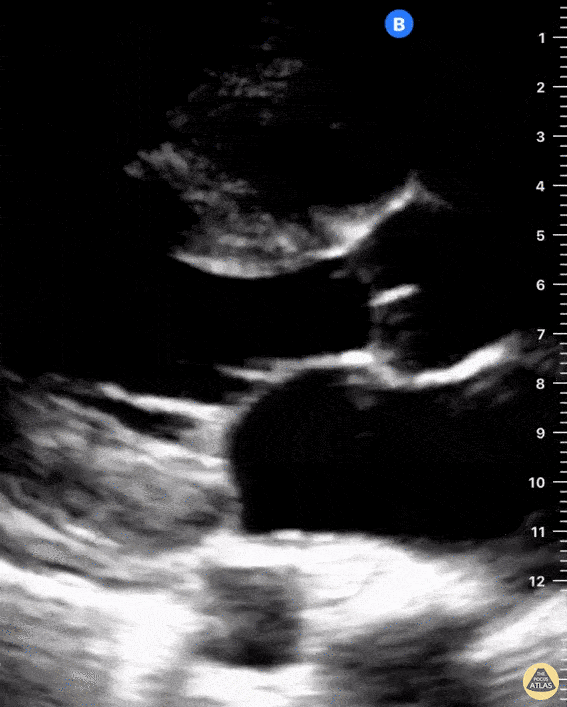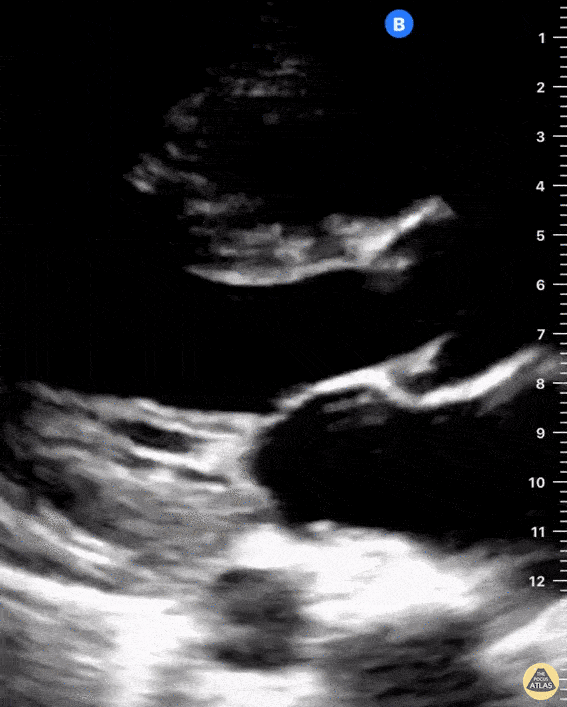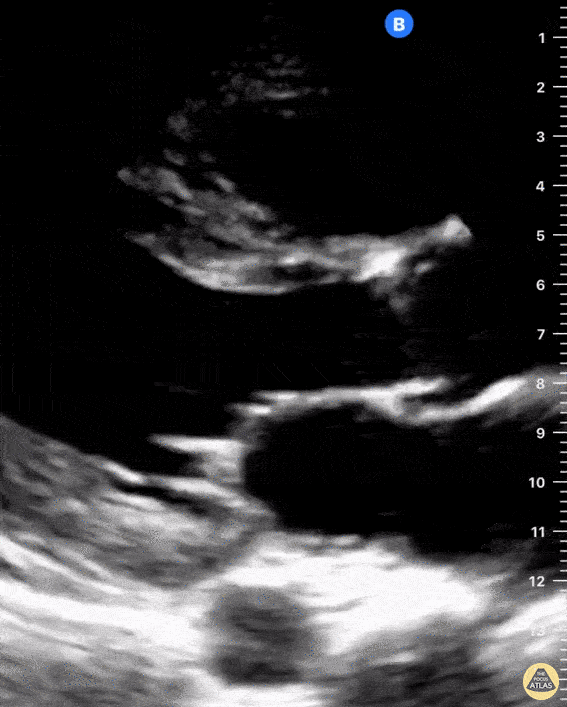
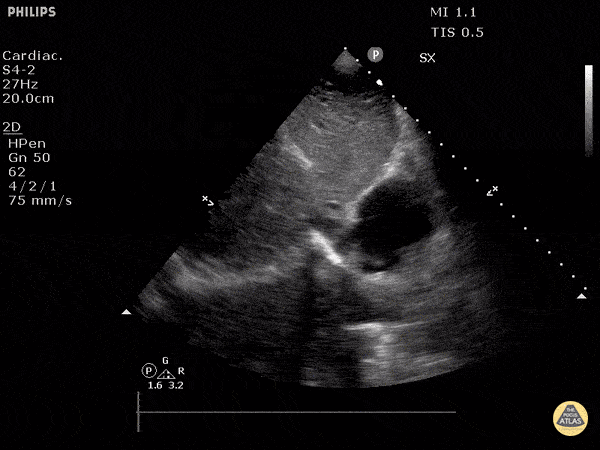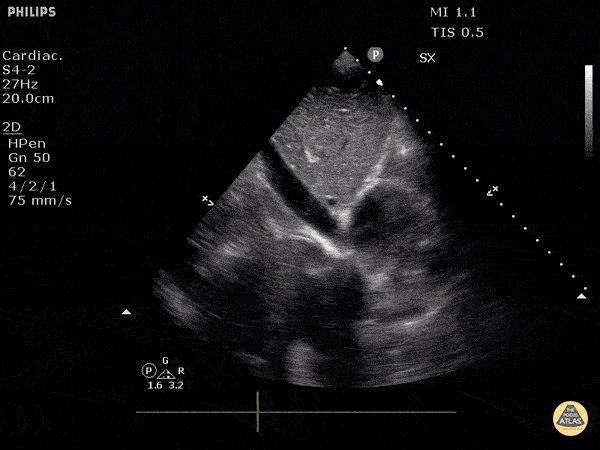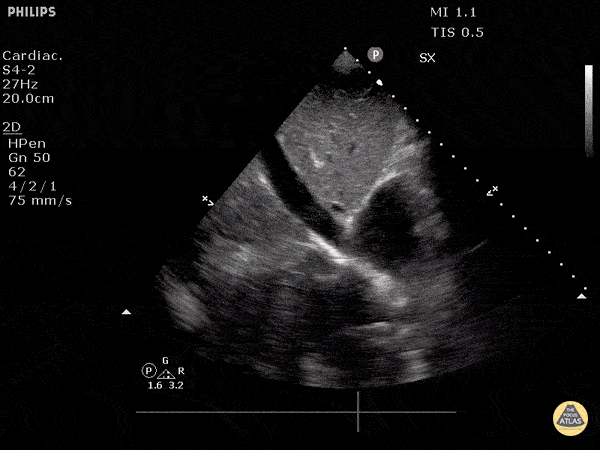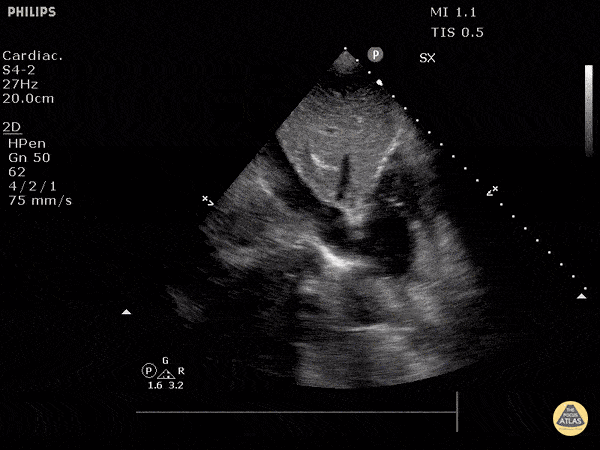
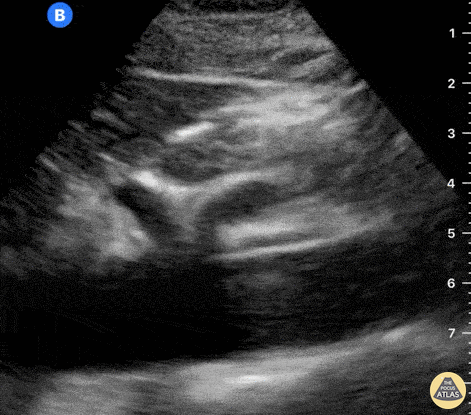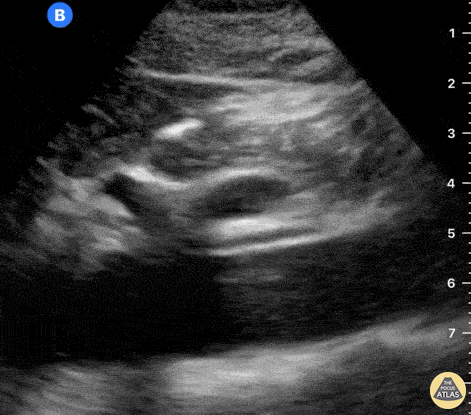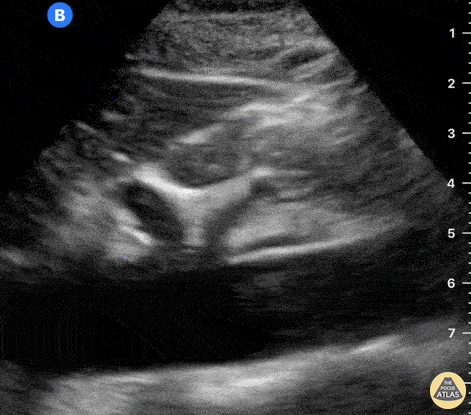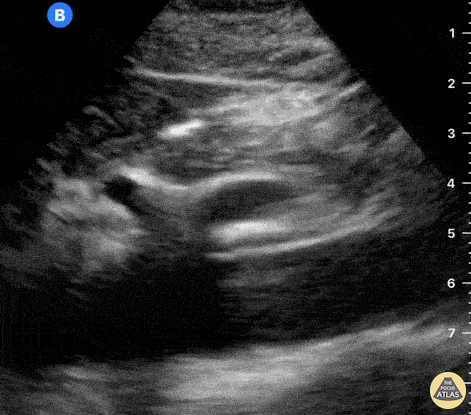
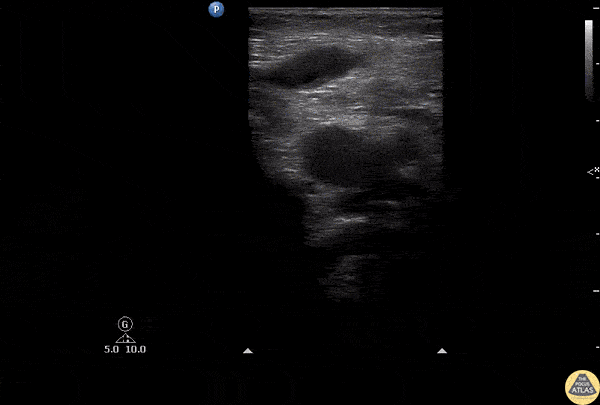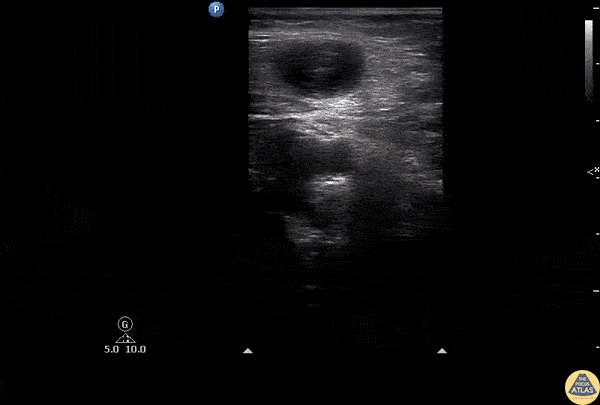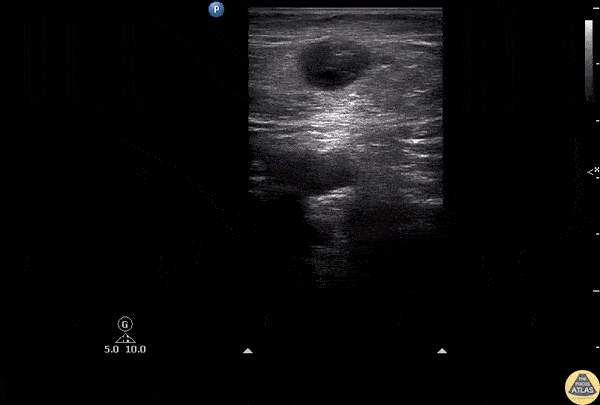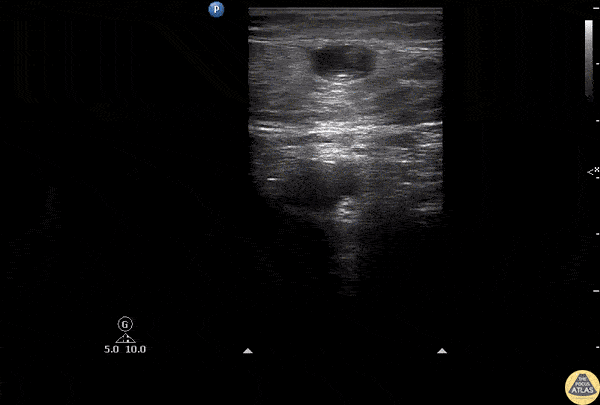
The right ventricle can also be evaluated using the same cardinal cardiac views discussed in the earlier section. Normally, the right ventricle is a low pressure chamber and appears small compared to its left sided counterpart. In right ventricular dysfunction, the main findings we are looking for are RV dilation and septal bowing. On the short axis, as the right ventricle dilates from increased pulmonary artery pressures this will lead to bowing of the inter-ventricular septum into the left side of the heart. This results in a D-shaped appearance of the left ventricle (D-sign). In the apical four chamber views, the right ventricle should usually only be 2/3 the size of the left ventricle. If the chambers appear the same size, that is considered to be abnormal and dilated. Another finding to look for is known as McConnell’s sign which can be described as right ventricular free wall hypokinesis with apical sparing. A common pitfall is assuming signs of right ventricular dysfunction are from an acute process such as pulmonary embolism. Unfortunately the findings discussed above can be both acute and chronic. Clinical context and patient history can be helpful in possibly teasing these out. A prior echo report can also be helpful to compare to your beside examination. Be careful when acquiring the apical four chamber view. If you are off axis, you can foreshorten the right ventricle and miss right ventricle dilation. To avoid this common error, rotate through the image to evaluate when the right heart is at its largest size. There are many other ways to evaluate right heart function including right atrial enlargement and TAPSE that are beyond the scope of this discussion.
Examples of Right Ventricular Dysfunction:
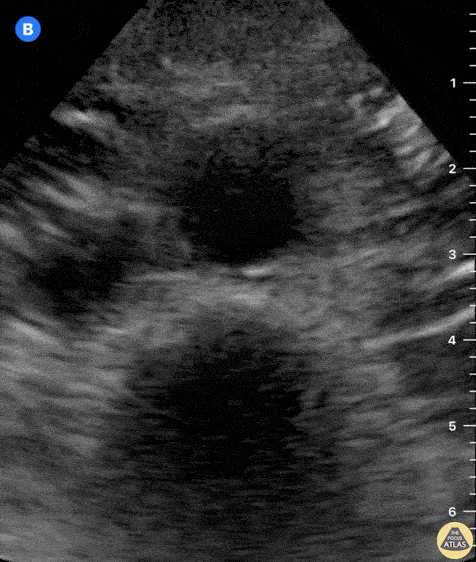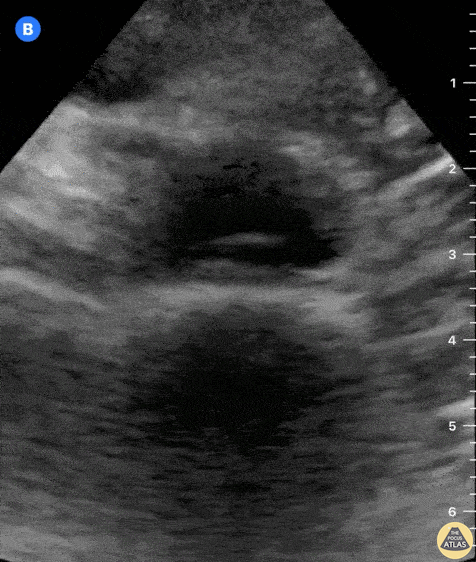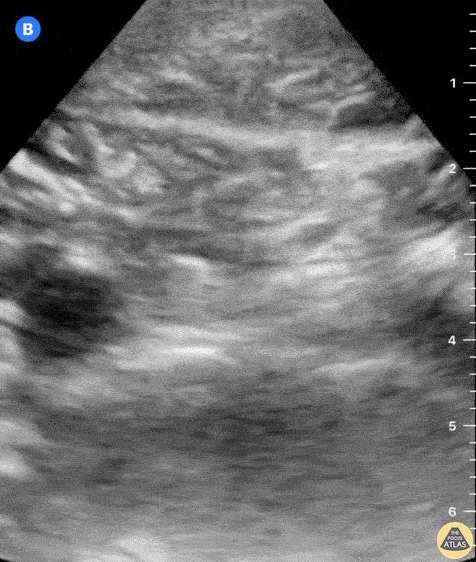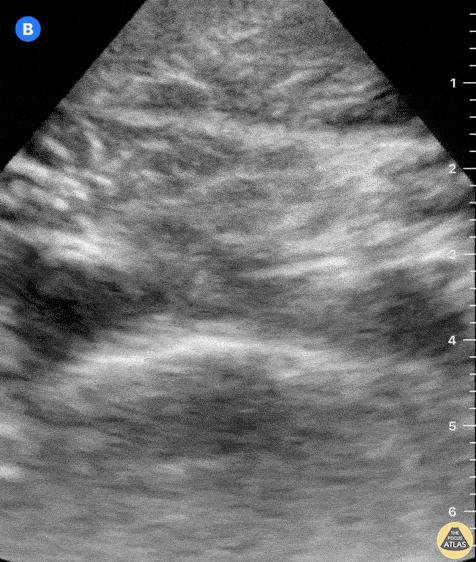
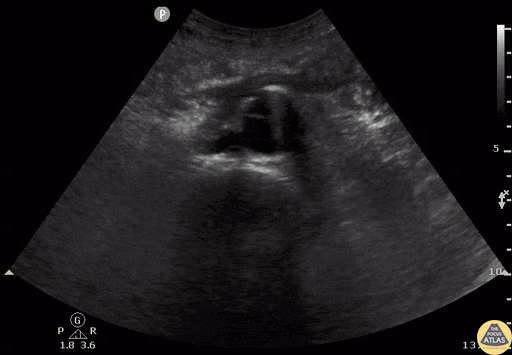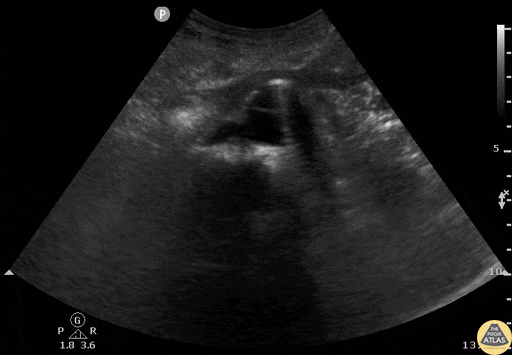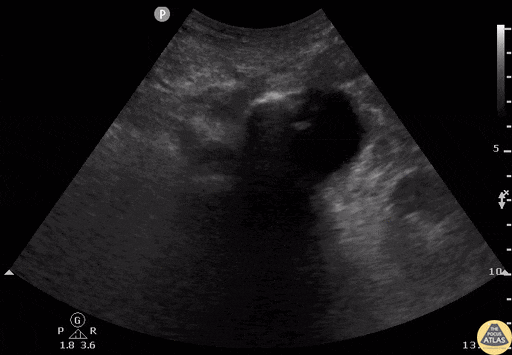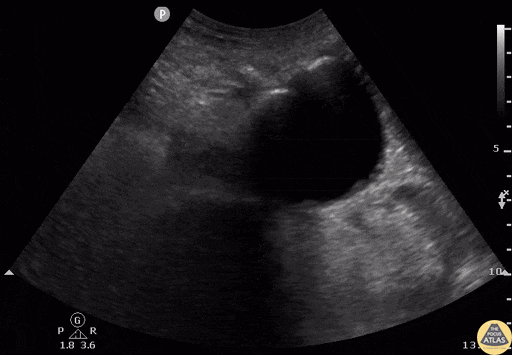
Tamponade
The presence of a circumferential effusion suggests possible tamponade and right ventricular end diastolic collapse confirms the diagnosis. Remember that an effusion on its own does not necessarily represent tamponade. Pay attention to right ventricular diastolic collapse (most specific finding) or right atrial systolic collapse (most sensitive finding) to help inform the situation. When tamponade is suspected, the diagnosis can be further supported by a plethoric IVC, which results from decreased venous return into the right atrium due to high pericardial pressure. Thus a collapsed IVC in the presence of an effusion makes tamponade unlikely unless a patient concurrently has significant volume depletion.
HI-MAP: IVC
The inferior vena cava (IVC) views supplement the cardiac views and should be a routine part of every bedside echo assessment. IVC size and respirophasic variations are used to estimate right atrial pressure and RV pre-load. IVC evaluation can be simplified into three categories:
Normal
Plethoric
Collapsed
Normal
An IVC with maximal diameter between 1.5 and 2 cm that collapses with respiratory variation is normal or sometimes indeterminate. Though cardiogenic and obstructive shock may be slightly less likely, they cannot be ruled out. Likewise, hypovolemic and distributive shock may also be present and cannot be ruled out by a single sonographic image without clinical context.
Plethoric
A plethoric IVC will usually measure > 2 cm in maximal diameter and will demonstrate minimal to no respiratory variation. A plethoric IVC can be indicative of right or left ventricular failure, an obstructive process (i.e. cardiac tamponade, massive pulmonary embolism, or tension pneumothorax), or volume overload.
Collapsed
A collapsed IVC is usually < 1.5 cm in maximal diameter and will completely or almost completely collapse during respiratory variation. In the context of hypotension, this is generally interpreted as a preload or volume deficiency that may be more consistent with hypovolemic or distributive shock states.
Pitfalls
Make sure to measure the IVC and not the aorta. They lie very close together so the best bet is to watch the IVC empty into the right atrium. Additionally, the hepatic veins draining into the IVC can also help to differentiate it from the aorta.
Measuring the IVC too close to the diaphragm can give you an inaccurate measurement as the diaphragm will tether open the IVC. The IVC should be evaluated ~ 2 cm distal to the right atrium.
If you come off axis of the IVC, it can look artificially narrow, so make sure it is measured at the widest point. This can also be avoided by performing evaluation of the IVC in a transverse orientation.
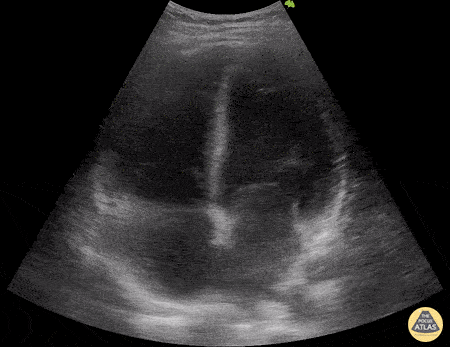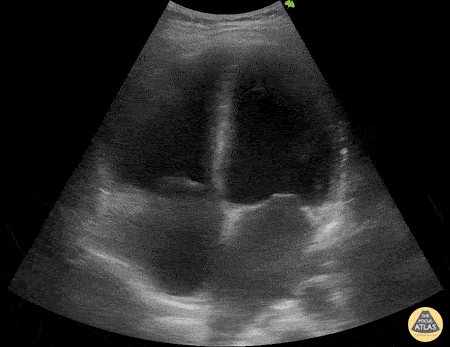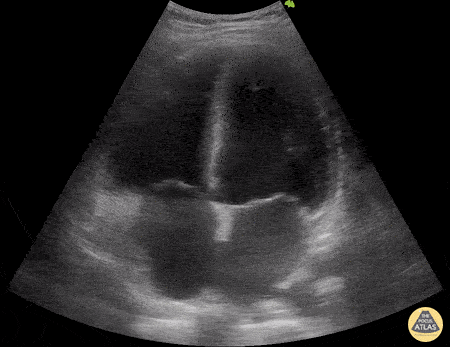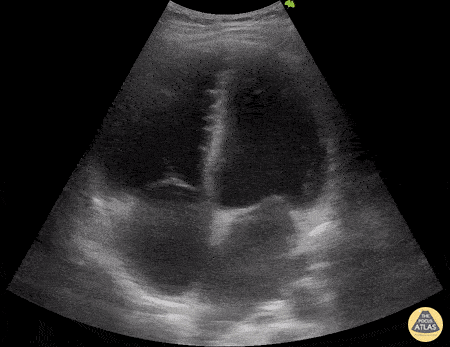
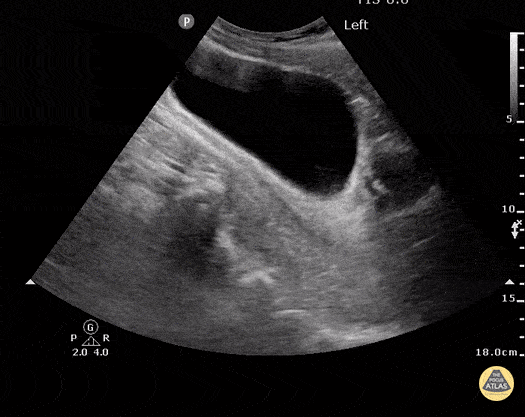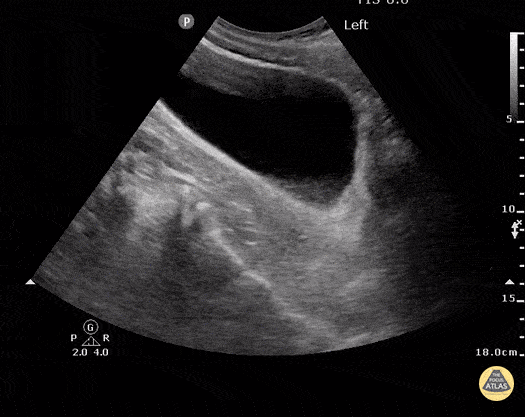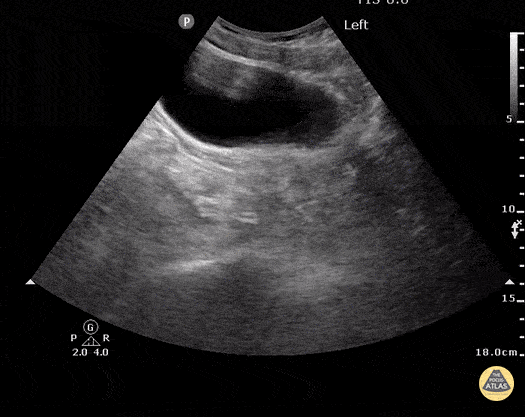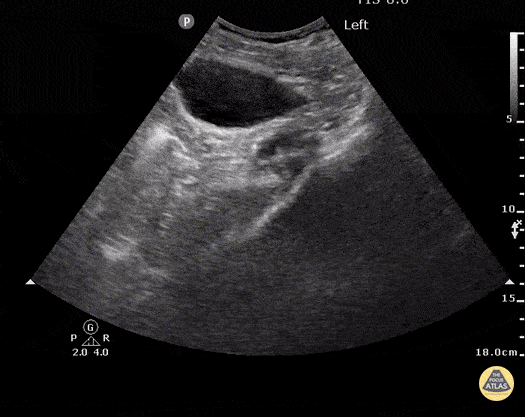
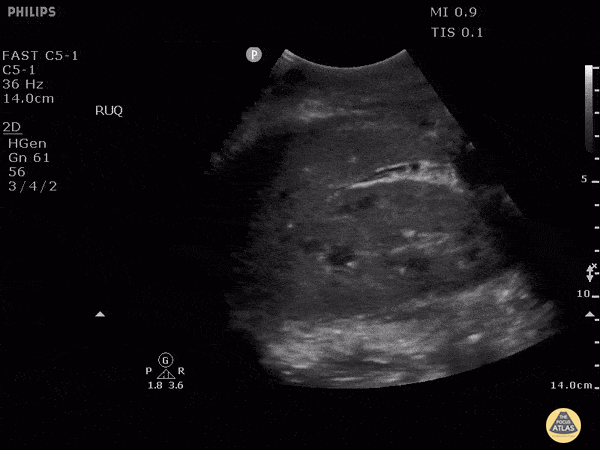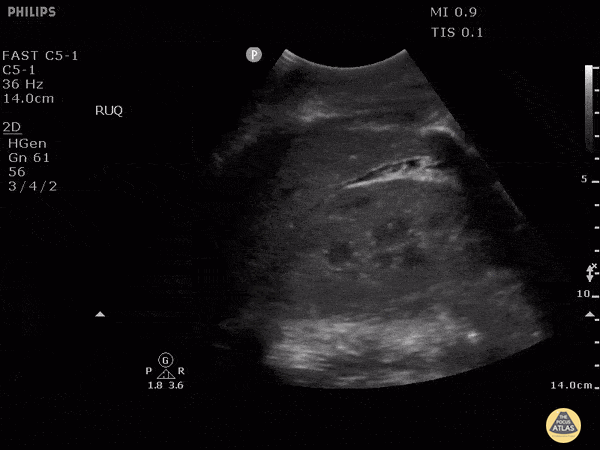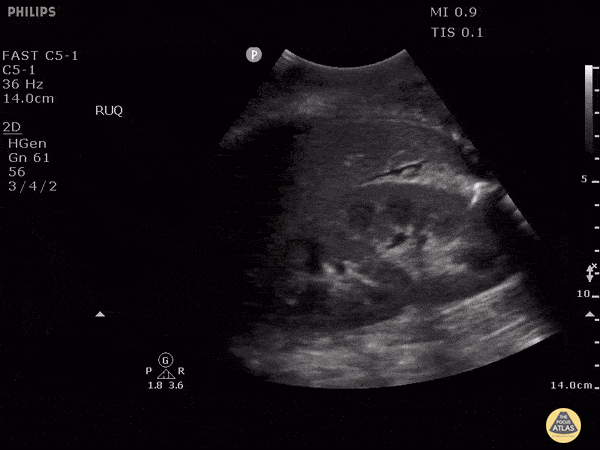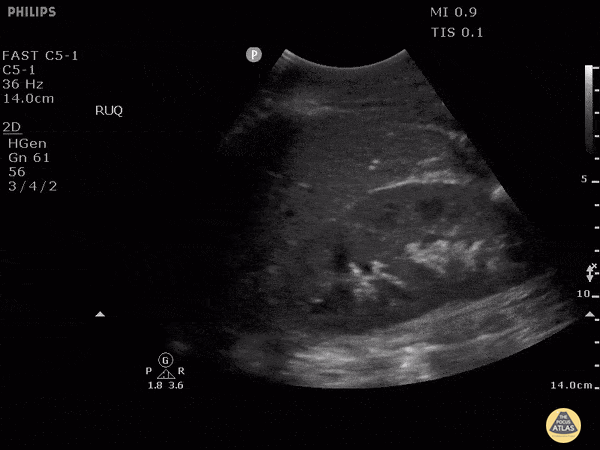
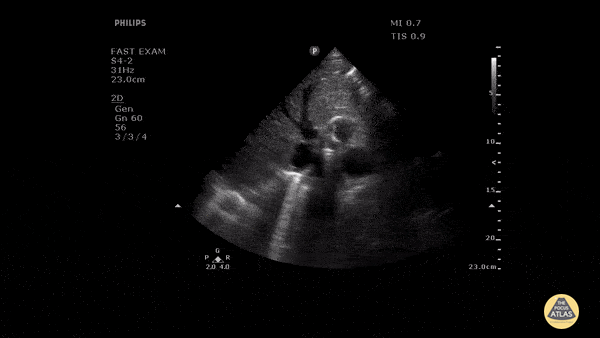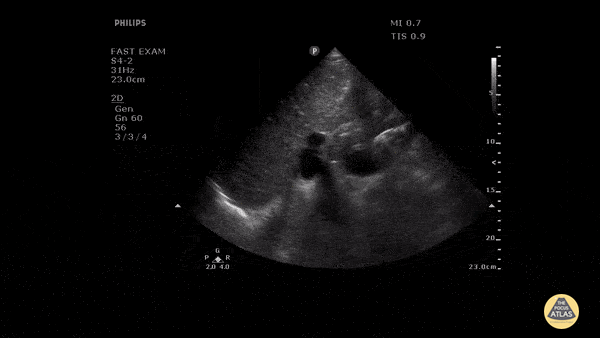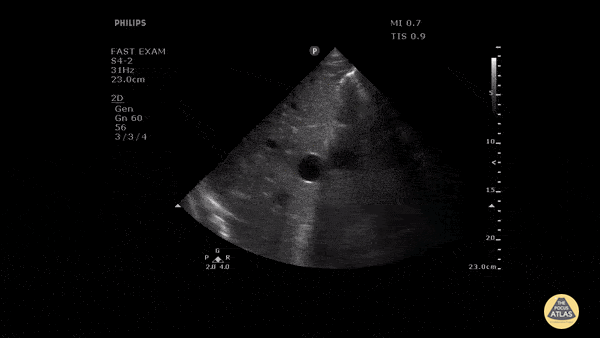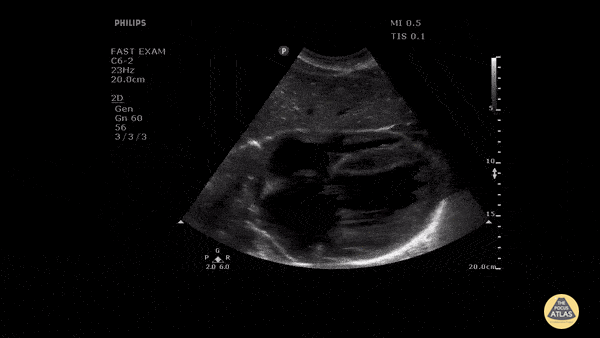
HI-MAP: Morison’s Pouch (FAST)
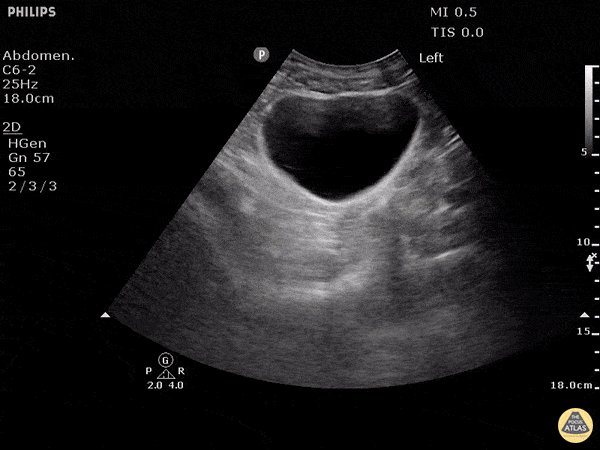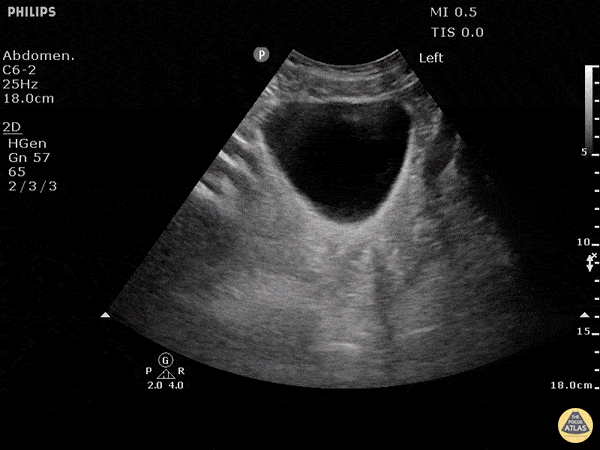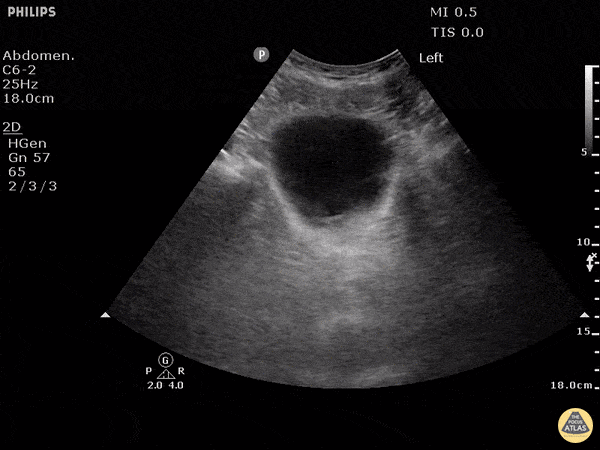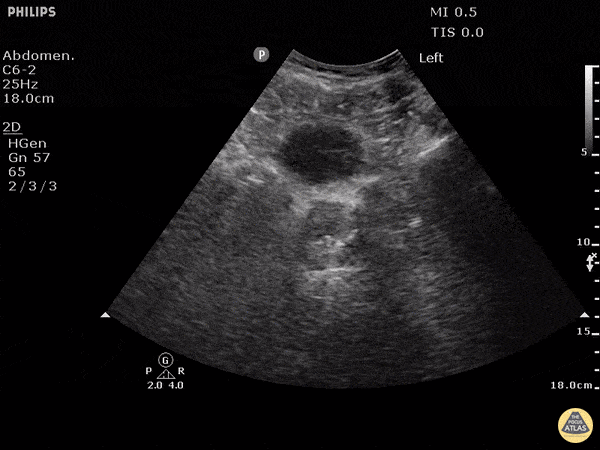
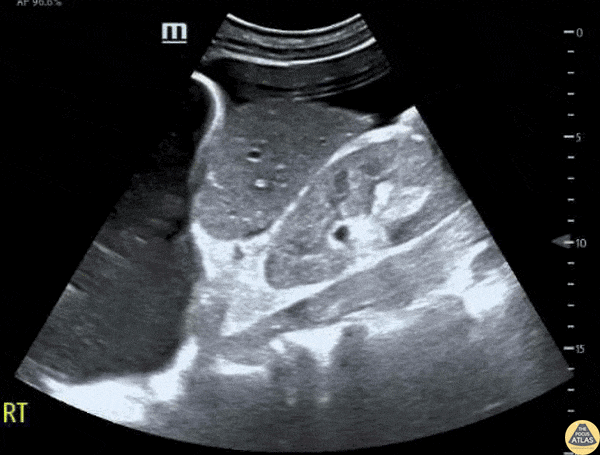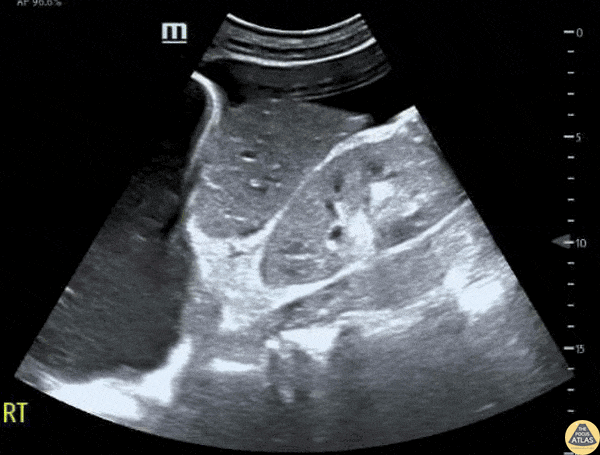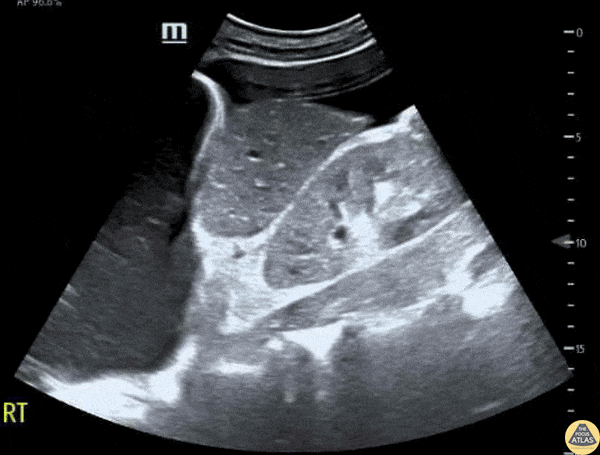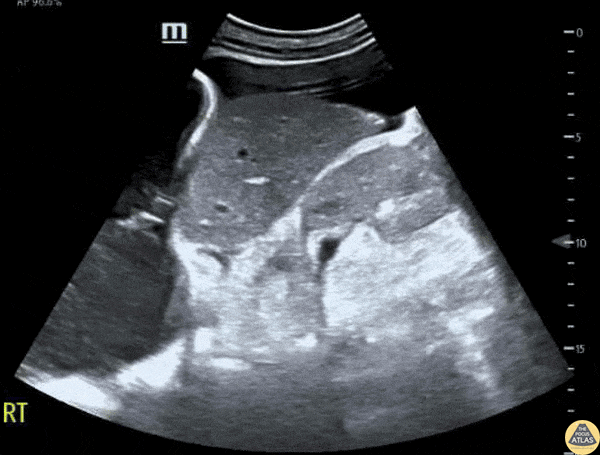
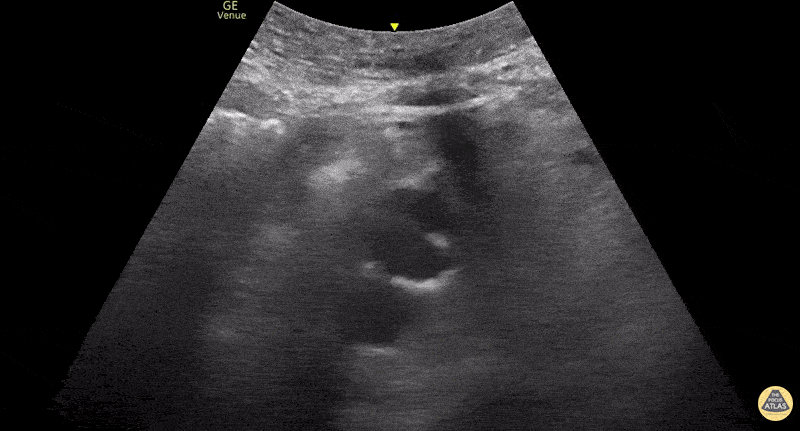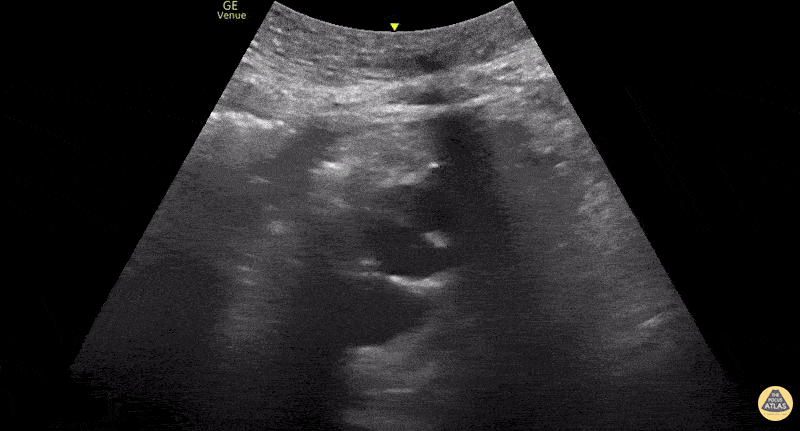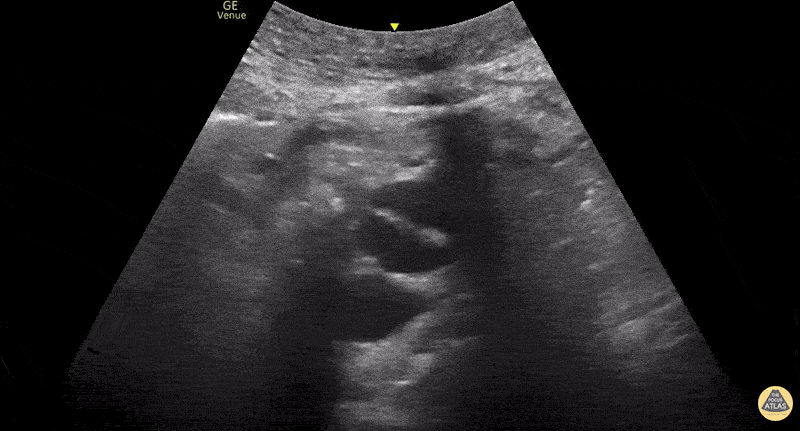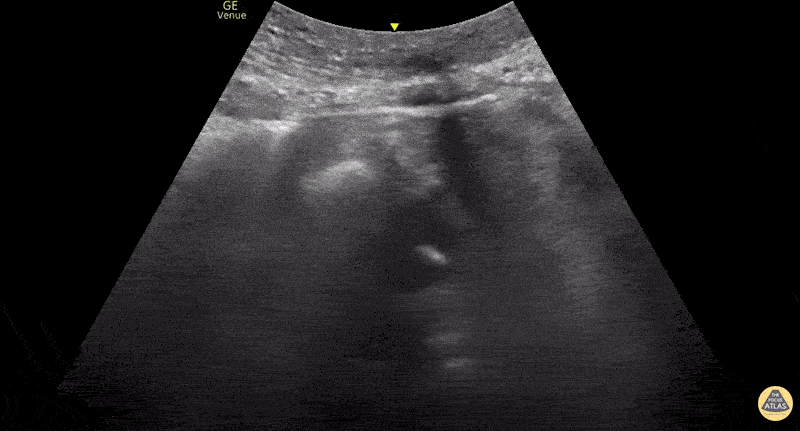
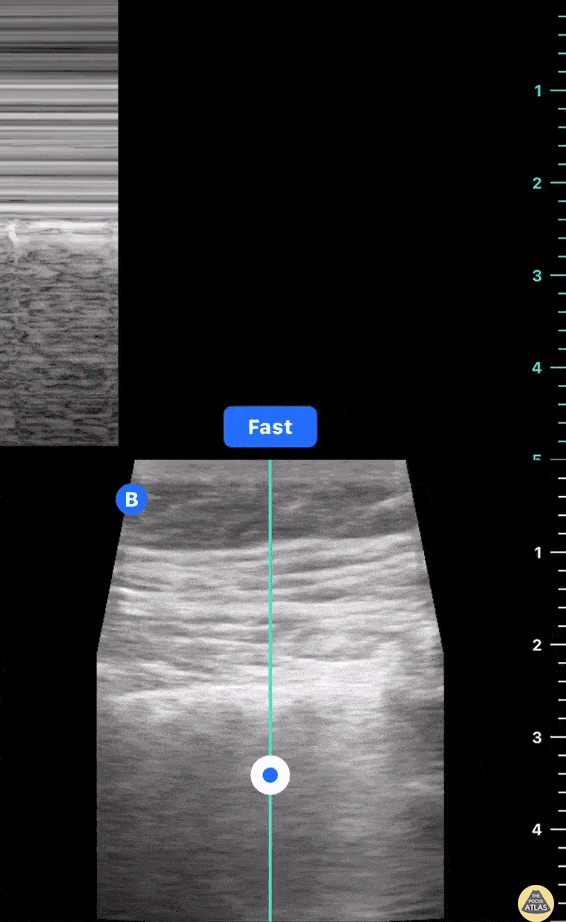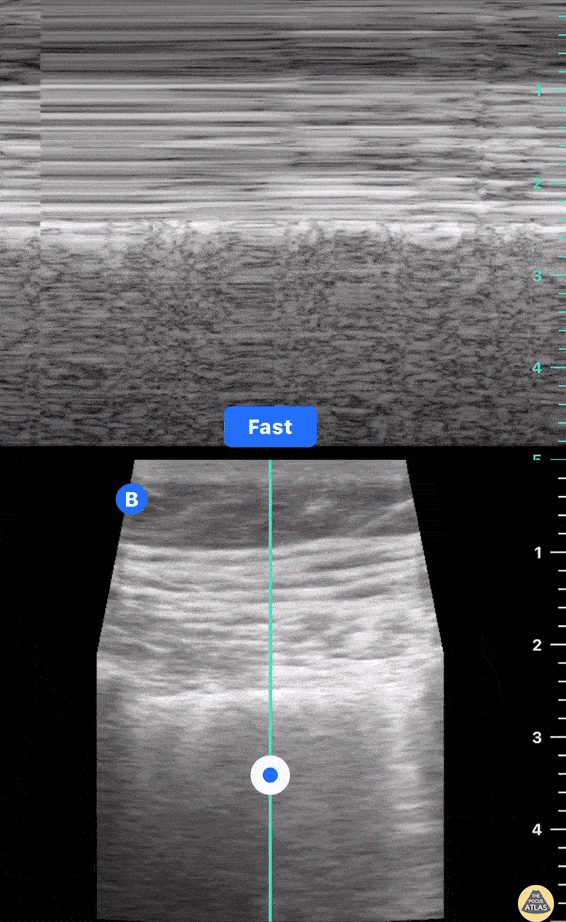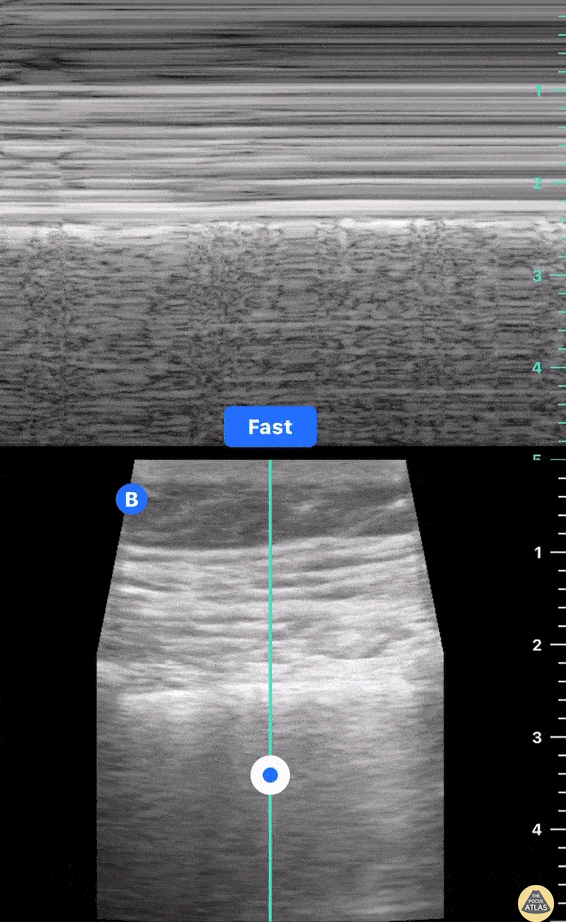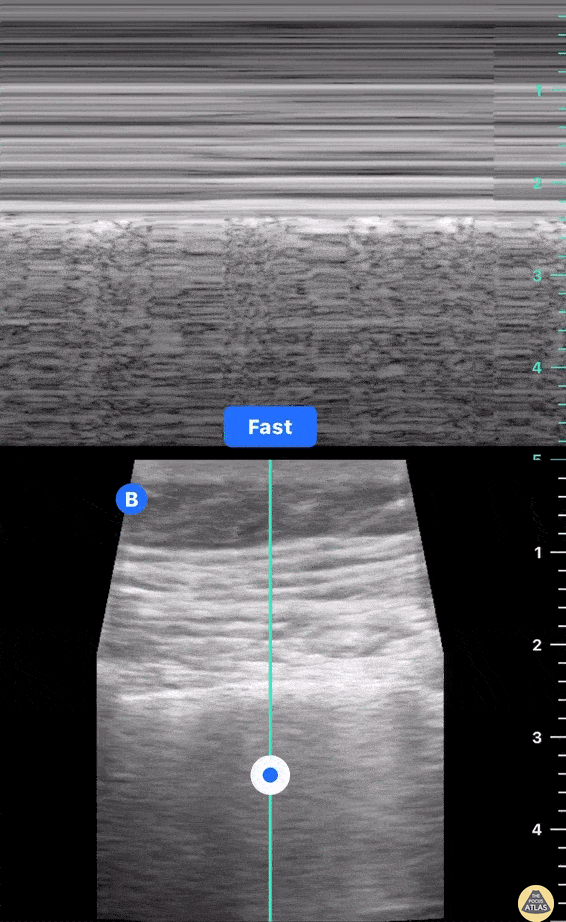
This exam has become ubiquitous for the assessment of trauma patients. However when used for the patient with unexplained shock, it can detect medical etiologies of intra-peritoneal hemorrhage as well. In addition to traumatic injuries, possible etiologies of intra-peritoneal hemorrhage include ruptured ectopic pregnancies, rupture of intra-abdominal blood vessels, aneurysms, bleeding tumors, and surgical complications. A small minority of abdominal aortic aneurysms rupture anteriorly into the peritoneum, so these views should not be used to rule out a ruptured abdominal aortic aneurysm (AAA). Remember you are looking for hypoechoic or anechoic fluid. Hypoechoic fluid is presumed to be blood in the peritoneum in the context of a hypotensive trauma patient. For more coverage of the FAST Exam, please see our Trauma Image Atlas.
Normal FAST Exam
Abnormal (Positive) FAST Exams
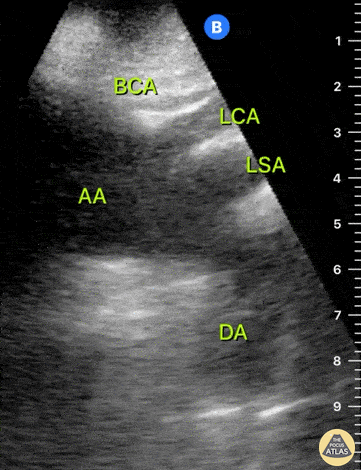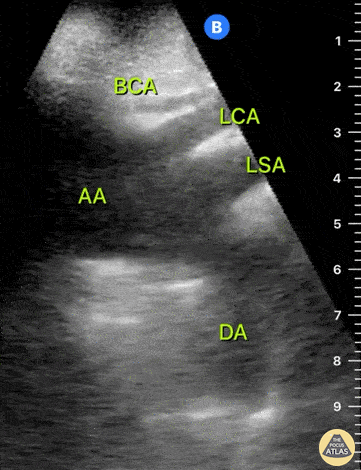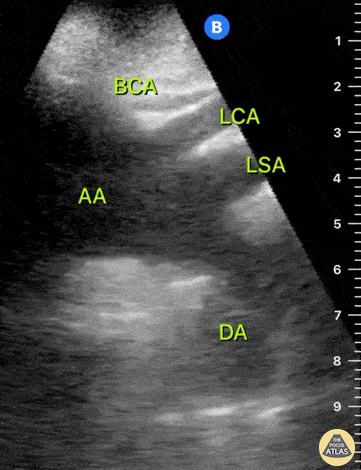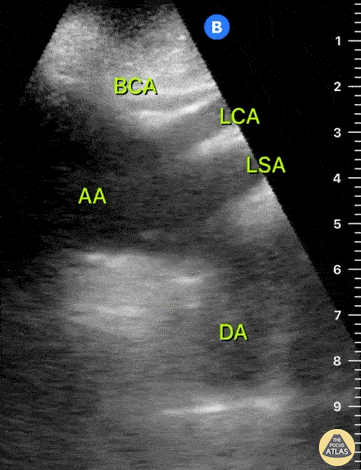
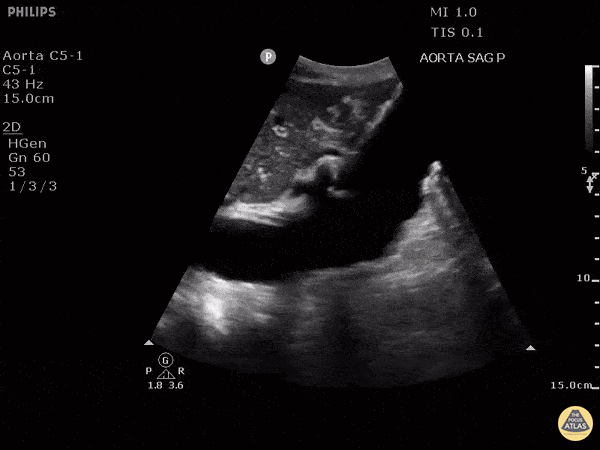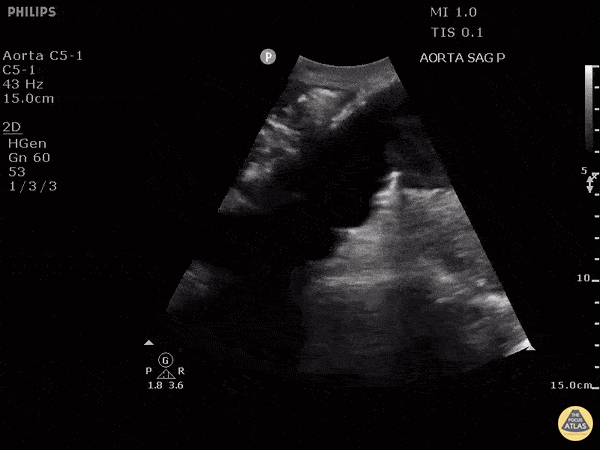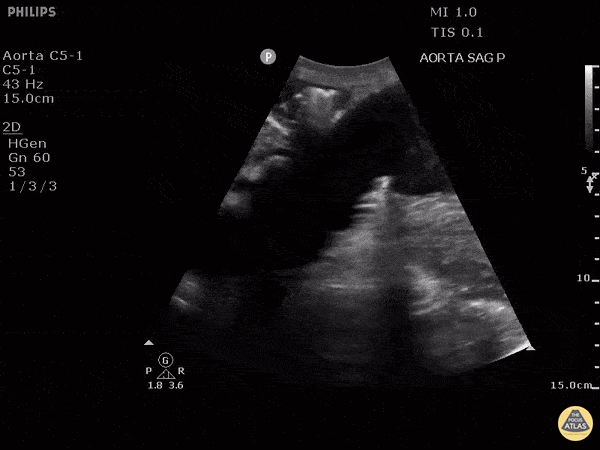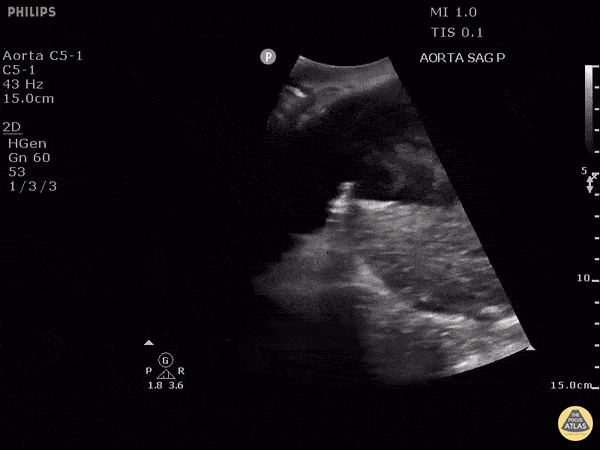
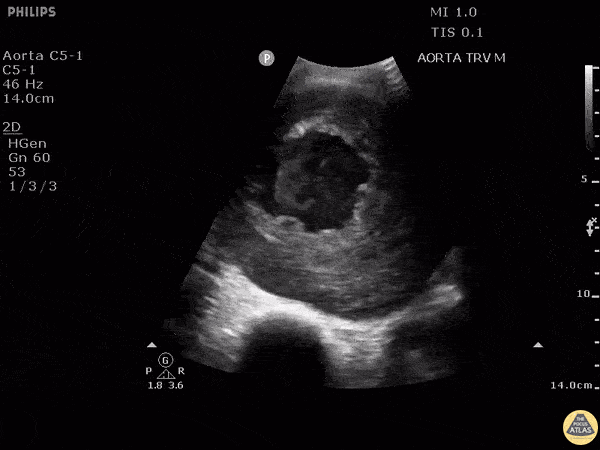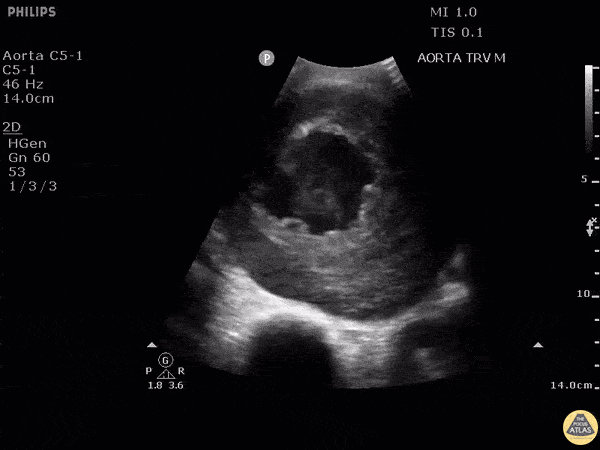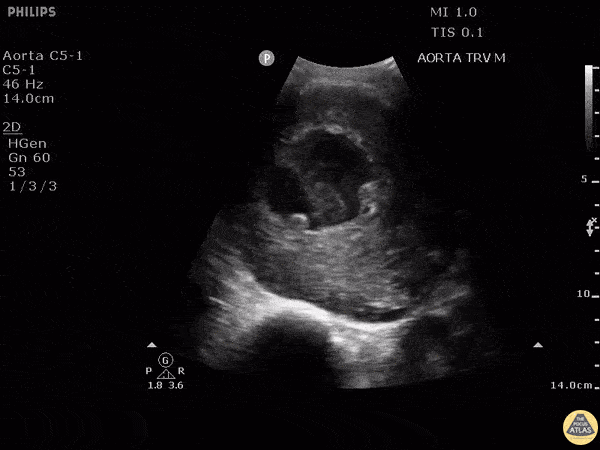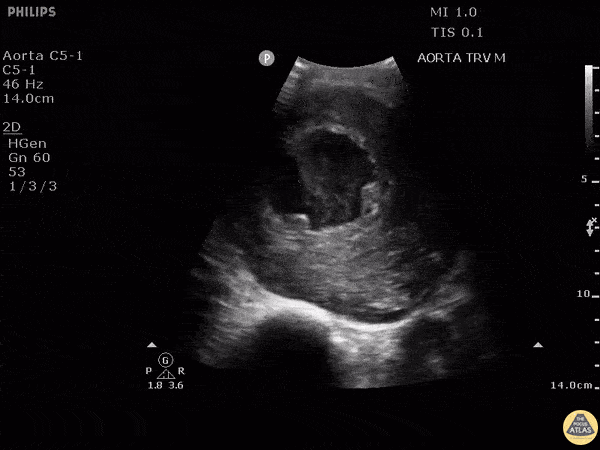
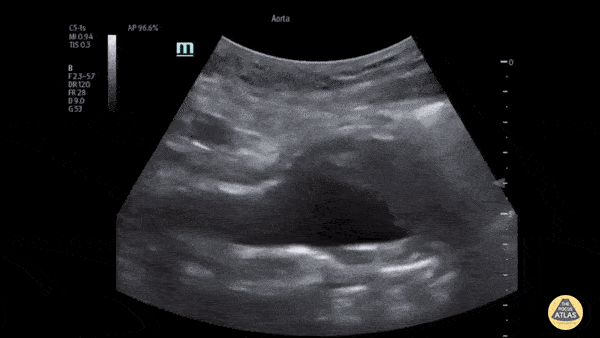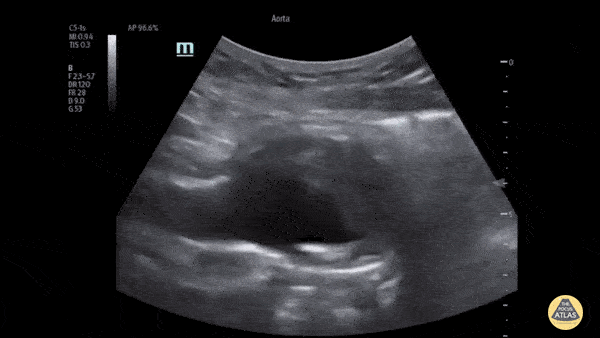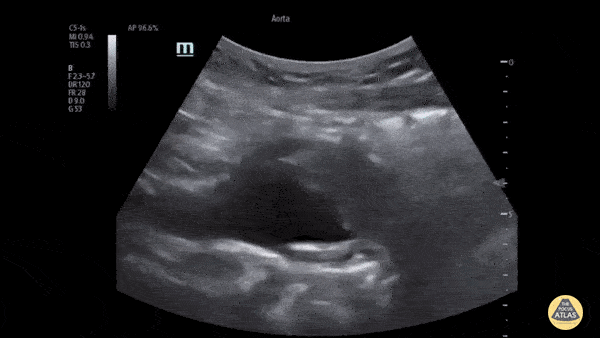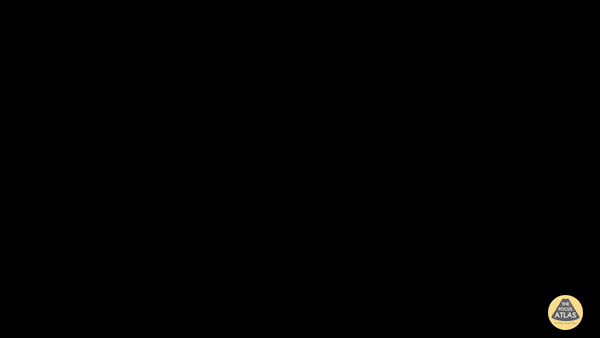
HI-MAP: Aorta:
Aorta pathology should be considered in the undifferentiated shock patient with abdominal or back pain (10). Typically this means ruling out a large abdominal aortic aneurysm (AAA). A normal aorta measures <3cm and any aorta that measures >5cm at any level warrants consideration of acute rupture in the appropriate clinical context. Views of the aorta should at least be obtained at 4 levels with emphasis on the infra-renal aorta as this is the most prevalent location for AAA. Ideally, the entire aorta can be imaged continuously to ensure no pathology along the entire course, but this is not always possible. Measurements are done in the transverse orientation, best performed with a curvilinear probe, and measured from outer wall to outer wall. Measurements are obtained including both outer walls because AAA and dissections can exist within the walls by definition, therefore our measurements must include both walls. It is best to image in both transverse and longitudinal planes as small saccular aneurysms can be missed if only scanned in one plane. It is crucial to note that the majority of ruptured AAA will not have intra-peritoneal free-fluid. Abdominal aortic aneurysms typically rupture into the retroperitoneum and this extravasated blood cannot readily be evaluated by ultrasound.
Normal Aorta:
Aortic Pathology:
While POCUS is not the standard imaging modality for dissection, dissection flaps can be visualized with ultrasound and should be noted when present, with the understanding that a normal ultrasound does not rule out this diagnosis.
Pitfalls:
Intra-aortic thrombus can be difficult to discern and can appear as a normal-caliber aorta leading to a false negative scan of the aorta.
Measurements made in the longitudinal plane may be off-center leading to a false negative scan. Measure in the transverse plane to ensure evaluation at the point of maximum diameter.
Bowel gas can make image acquisition difficult. Position the patient with hips and knees flexed and apply steady pressure to the abdomen over time to allow gas to clear from view.
Make sure to measure the aorta and not the IVC. Both vessels are pulsatile, so ensure you are able to identify the celiac trunk, SMA, etc. that clearly identify the abdominal aorta.
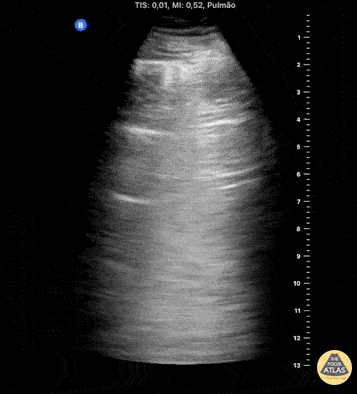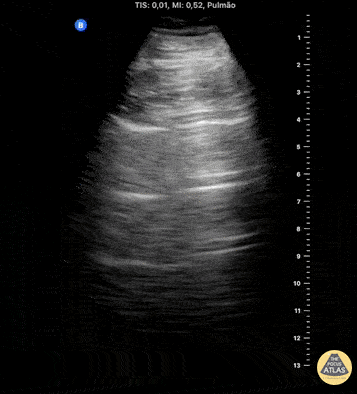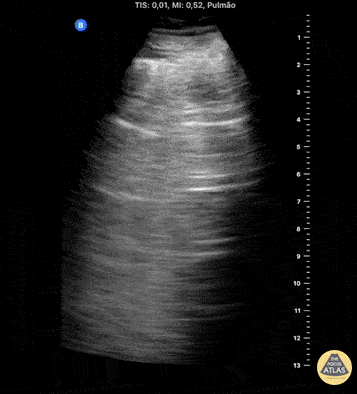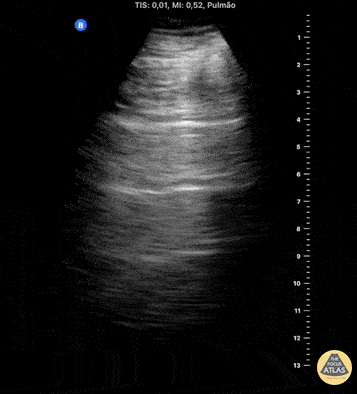
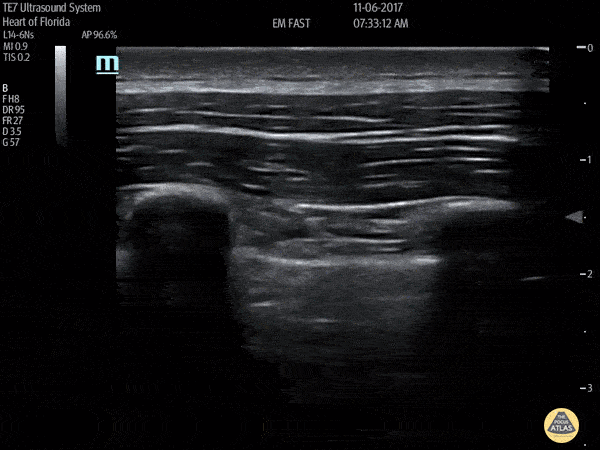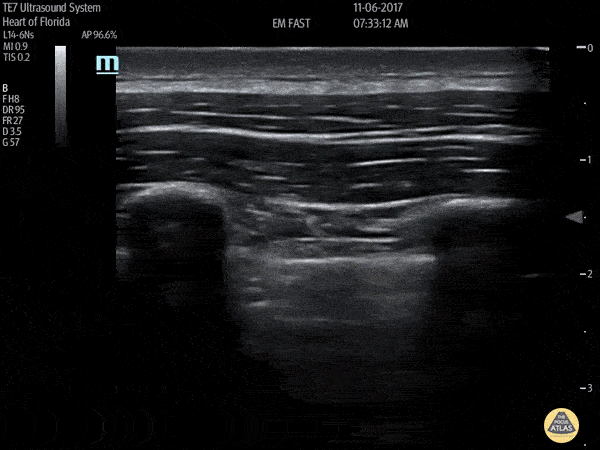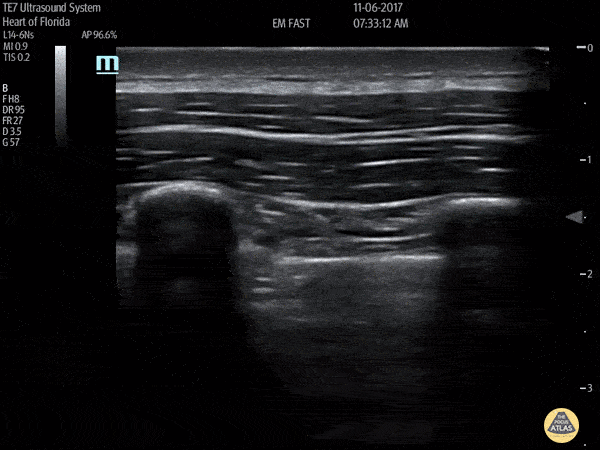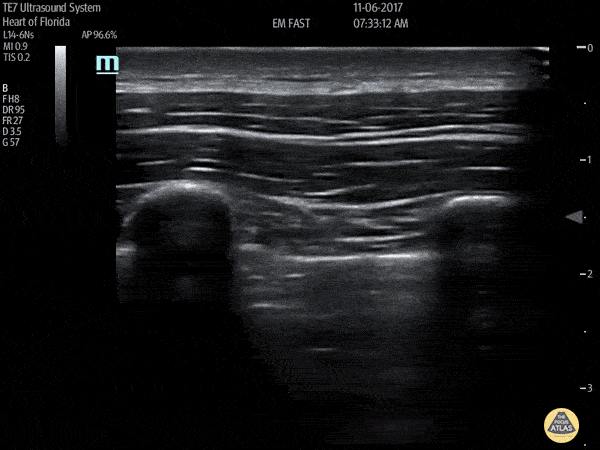
HI-MAP: Pneumothorax/Pulmonary
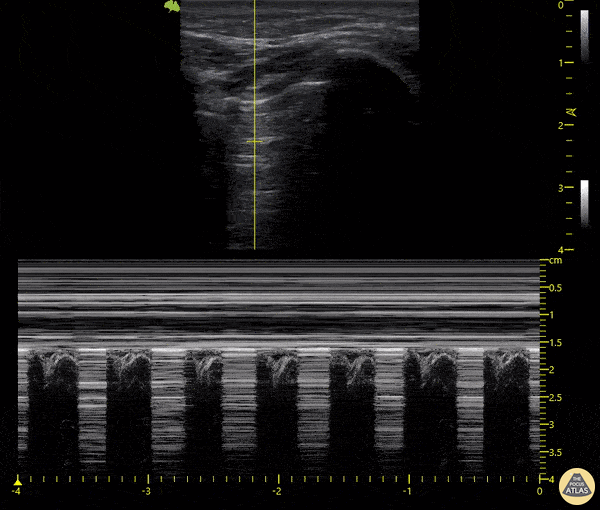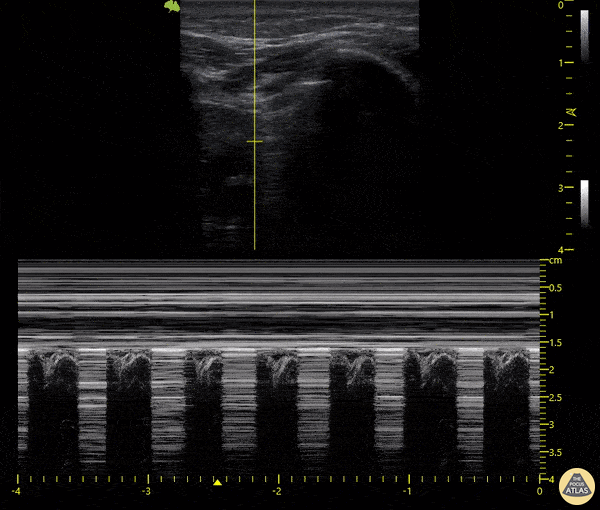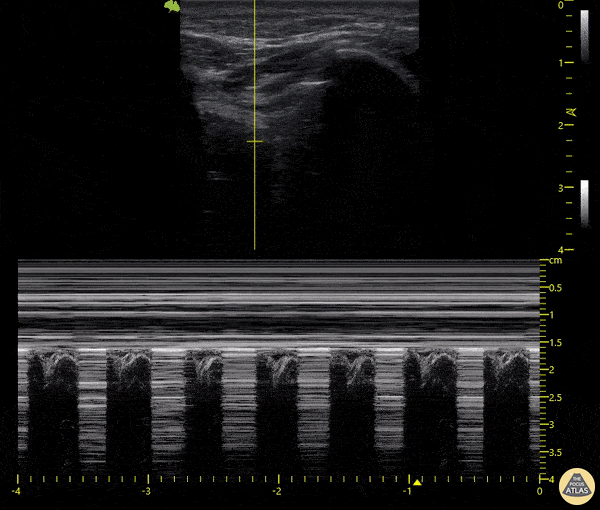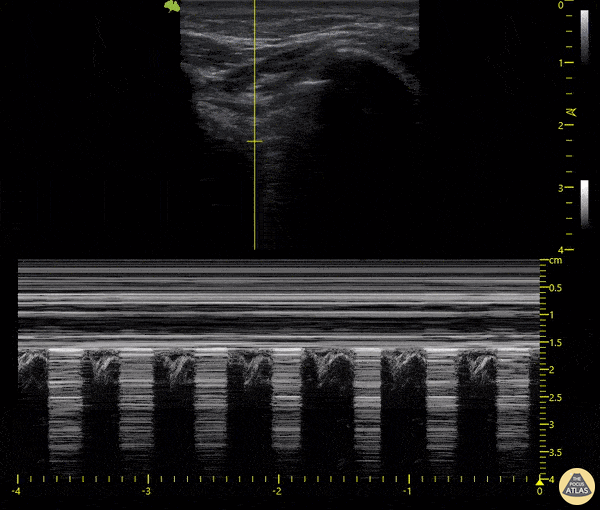
The main consideration in pulmonary ultrasound in critically ill patients is the presence of a pneumothorax. As the patient is likely supine, image in the least dependent area (where air would rise). This is typically the anterior chest along the mid-clavicular line starting from the second intercostal space and imaging caudally. Use a high frequency probe in the sagittal orientation. Image bilaterally and assess for presence of lung slide and comet tail artifact. Presence of either confirms parietal pleura and visceral pleura are in contact, and thus, no pneumothorax. Lung sliding appears as a “shimmering” along the pleural interface (seen as a hyperechoic line) that is just deep to the visualized ribs. It is helpful to use the ribs to identify the pleural line.
Presence of lung slide bilaterally can exclude tension pneumothorax as an etiology of acute shock while absence of lung slide strongly suggests pneumothorax. Visualization of a lung point (the exact point where the the layers of pleura start to be separated by air) is diagnostic of pneumothorax.
M-mode can also be used to evaluate for pneumothorax. Using the same view obtained to evaluate for lung sliding, obtain a clip using M-mode. If lung sliding is present (i.e. no pneumothorax), a clear delineation will be seen at the level of the pleural interface, referred to as a “seashore” sign (pictured below). If there is no lung sliding (i.e. pneumothorax is suggested), no delineation is seen and there will be continuous lines from the top of the screen to the bottom, referred to as a “barcode” sign.
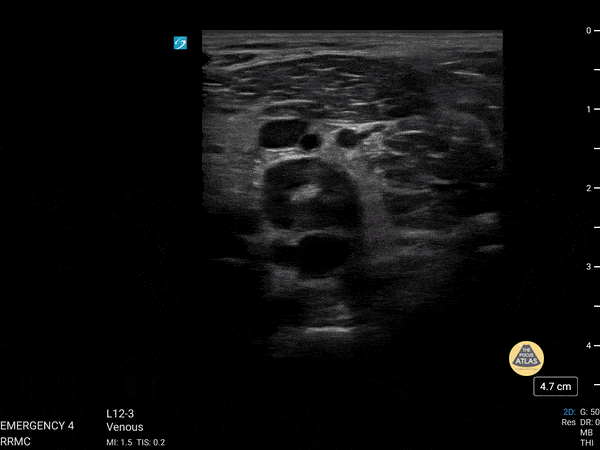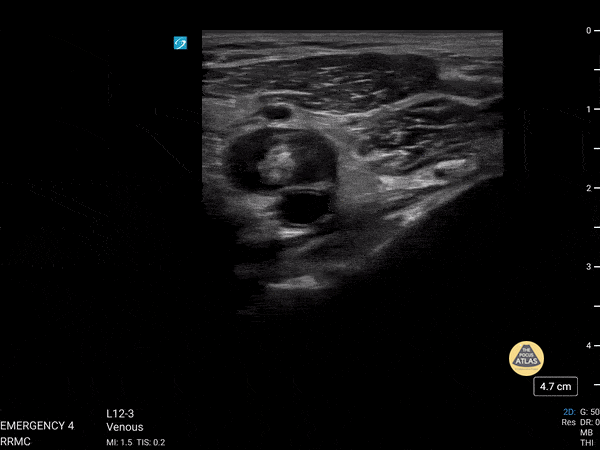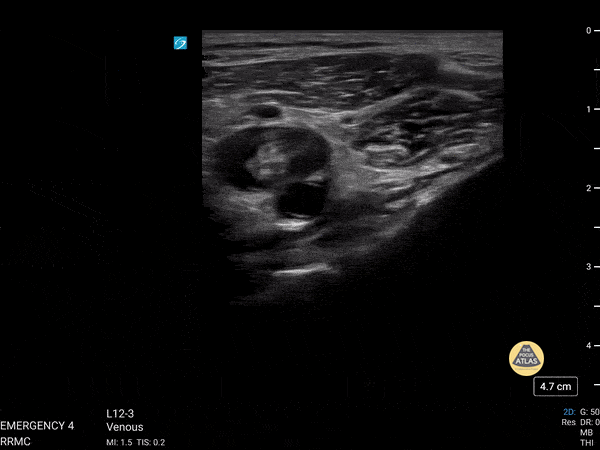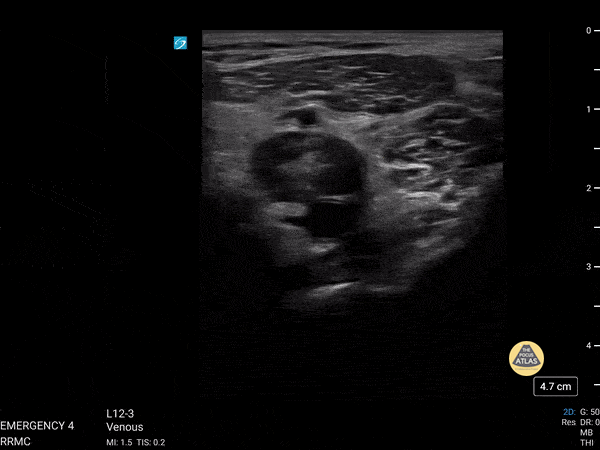
+/- DVT
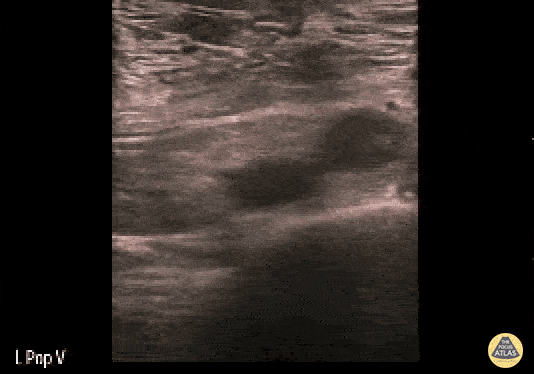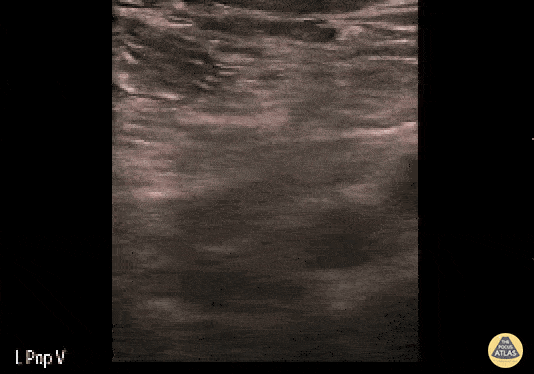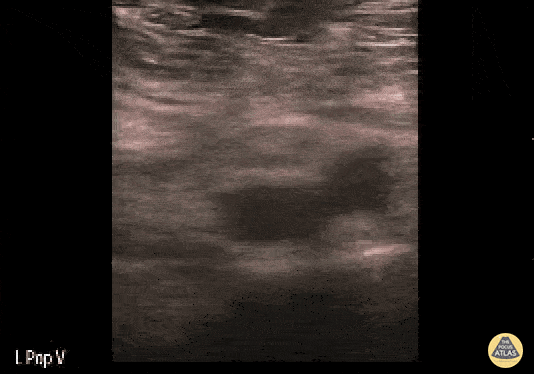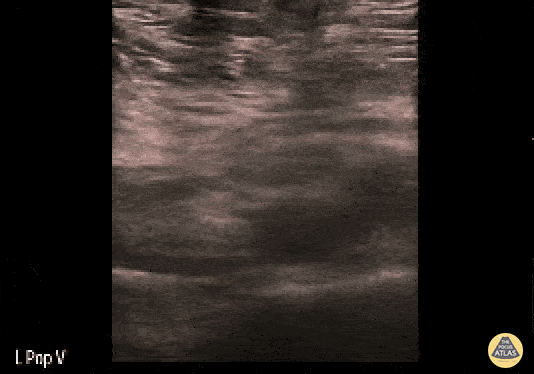
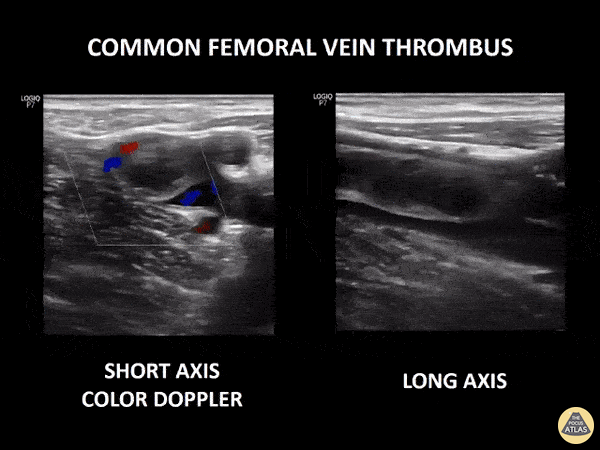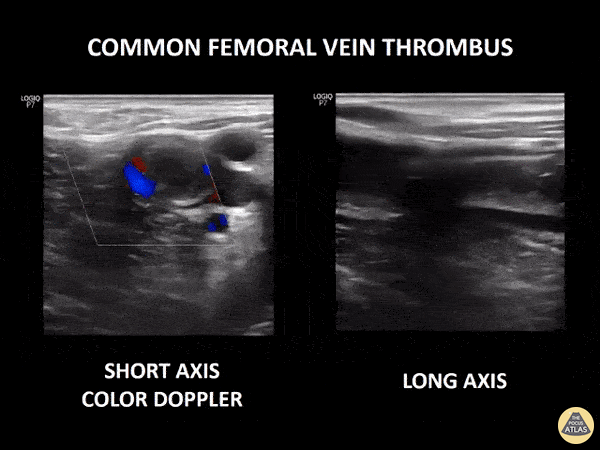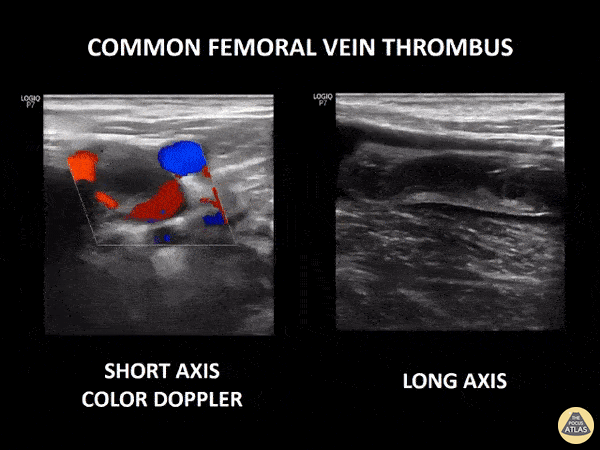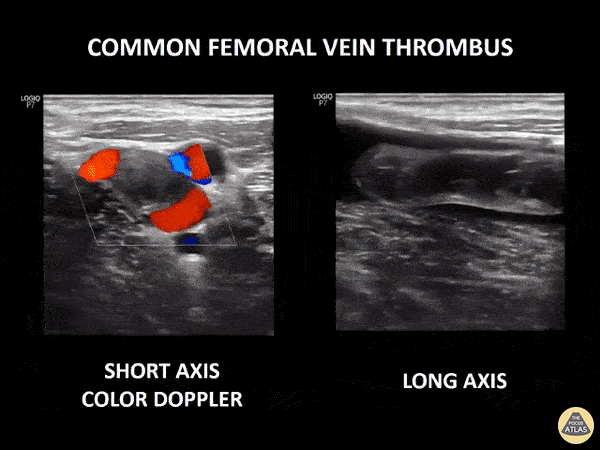
If during your assessment of the heart and IVC, you are concerned about massive pulmonary embolism (RV strain/dilation + plethoric IVC + clear lungs), consider evaluating the bilateral lower extremities for deep venous thrombosis (DVT). There is strong evidence that clinicians can rapidly and accurately assess for presence of DVT using POCUS with both high sensitivity and specificity (14). To assess the common femoral vein, begin by scanning just distal to the inguinal ligament. The vein is typically medial to the artery. Compression is applied until the artery is mildly compressed. If the vein is not completely compressible, this represents a positive study as seen below. When evaluating the femoral vein, ensure you are able to evaluate distal to the greater saphenous and common femoral branch point. Proximal saphenous vein clots (within 5cm of the femoral vein) are treated as DVTs so it is important to image the junction of saphenous and femoral vein (11). Additionally, evaluate for DVT at the branch point of the deep femoral from the common femoral vein. The popliteal vessels are then evaluated in a similar fashion. The popliteal vein sits superficial to the popliteal artery. As before, be sure to identify and evaluate the branch point of the anterior and posterior tibial veins as well as the fibular (peroneal) vein.
DVT Pathology:
Summary:
Ultrasound is helpful to evaluate patients presenting with signs of shock or critical illness in almost any clinical scenario
Ultrasound can help to rapidly narrow your differential diagnosis
Most views required are already used in other studies and easy to learn
You do not need to obtain every view, tailor your exam to the clinical context
Always take into account the clinical history of the patient and interpret your ultrasound findings in light of this context
References:
Sasmaz MI, Gungor F, Guven R, Akyol KC, Kozaci N, Kesapli M. Effect of Focused Bedside Ultrasonography in Hypotensive Patients on the Clinical Decision of Emergency Physicians Emergency Medicine International. 2017; 2017:1-8.
Stickles SP, Carpenter CR, Gekle R, et al. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: A systematic review and meta-analysis CJEM. 2019; 21(3):406-417.
Keikha M, Salehi-Marzijarani M, Soldoozi Nejat R, Sheikh Motahar Vahedi H, Mirrezaie SM. Diagnostic Accuracy of Rapid Ultrasound in Shock (RUSH) Exam; A Systematic Review and Meta-analysis. Bull Emerg Trauma. 2018; 6(4):271-278. [PDF]
Jones AE, Tayal VS, Sullivan DM, Kline JA. Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004; 32(8):1703-8.
Atkinson PRT, McAuley DJ, Kendall RJ, et al. Abdominal and Cardiac Evaluation with Sonography in Shock (ACES): an approach by emergency physicians for the use of ultrasound in patients with undifferentiated hypotension Emergency Medicine Journal. 2009; 26(2):87-91.
Galaz Tavares J, Ivo R, Gonzalez F, Lamas T, Mendes JJ. Global Ultrasound Check for the Critically lll (GUCCI)—a new systematized protocol unifying point-of-care ultrasound in critically ill patients based on clinical presentation OAEM. 2019; Volume 11:133-145.
McKaigney CJ, Krantz MJ, La Rocque CL, Hurst ND, Buchanan MS, Kendall JL. E-point septal separation: a bedside tool for emergency physician assessment of left ventricular ejection fraction The American Journal of Emergency Medicine. 2014; 32(6):493-497.
Farsi D, Hajsadeghi S, Hajighanbari MJ, et al. Focused cardiac ultrasound (FOCUS) by emergency medicine residents in patients with suspected cardiovascular diseases. J Ultrasound. 2017; 20(2):133-138. [PDF]
Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic Review: Emergency Department Bedside Ultrasonography for Diagnosing Suspected Abdominal Aortic Aneurysm Acad Emerg Med. 2013; 20(2):128-138.
Blumenberg RM, Barton E, Gelfand ML, Skudder P, Brennan J. Occult deep venous thrombosis complicating superficial thrombophlebitis Journal of Vascular Surgery. 1998; 27(2):338-343.
Shokoohi H, Boniface KS, Pourmand A, et al. Bedside Ultrasound Reduces Diagnostic Uncertainty and Guides Resuscitation in Patients With Undifferentiated Hypotension* Critical Care Medicine. 2015; 43(12):2562-2569.
Atkinson PR, Milne J, Diegelmann L, et al. Does Point-of-Care Ultrasonography Improve Clinical Outcomes in Emergency Department Patients With Undifferentiated Hypotension? An International Randomized Controlled Trial From the SHoC-ED Investigators Annals of Emergency Medicine. 2018; 72(4):478-489.
Lee JH, Lee SH, Yun SJ. Comparison of 2-point and 3-point point-of-care ultrasound techniques for deep vein thrombosis at the emergency department: A meta-analysis. Medicine (Baltimore). 2019;98(22):e15791.